The Top 3 "Dirty Little Secrets" Lurking In The Pain Relief Industry's Closet Of LIES!
Dear frustrated pain sufferer,
Why is it that you're forced to simply EXPECT a laundry-list of side effects if you start a new pain medication?
Why is it that millions of people every day are watching commercials where the list of side effects is longer than the commercial itself... yet STILL spend their hard-earned money on those products?
And why is it that millions of people all around the world are still willing to ACCEPT these dreadful, mind-fogging, life-shortening side effects even though there are safe alternatives available?

Hi, my name is Jesse Cannone.
I'm the founder of The Healthy Back Institute®, and in the past 10 years and with the help of my medical advisory board of 7 doctors and pain specialists... I've personally helped over 165,385 people in 85 countries free themselves of the shackles of back, neck and other pain.
And I've done so with all-natural, 100% safe, innovative and sometimes controversial pain relieving methods.
I've been featured in dozens of newspapers and magazines such as Entrepreneur Magazine, Women Today, Woman's World, and Visage... numerous radio programs... thousands of websites and even as a featured pain expert on NBC!
And in the letter below, I'd like to clue you in on the top three dirty little secrets the "pain relief" industry is trying to hide from your eyes. Secrets that are not only lining the pockets of "Big Pharma", but also KILLING people just like you as a result.
Secrets like...
- One of the most DEADLY, yet common pain medications on the market (Hint: If you're taking this silent killer, you could be subjecting yourself to a 340% increased risk of heart attack or stroke!)
- The scientific discovery made in 1972 which gave a scientific explanation as to why you experience more pain as you age... PLUS... how to easily and quickly FIX this problem without resorting to drugs or surgery!
- The 12 most powerful and safest anti-pain ingredients which not only decrease your pain but can also help reduce blood pressure... brighten and clear up your skin... give you a rock-solid immune system... cleanse and thin your blood... and much, much more!
Let's get started by giving you a sneak peek into the lives of people taking these life-sucking pain medications and why you should be running for the hills if you're currently taking any medications from major drug corporations.
Dirty Little Secret #1:
Drug Side Effects Are Not Only Dangerous...
They're DEADLY!
For years, even decades we've been told that if you have pain... just taking something like... Advil... Aleve... Bayer... Celebrex... Demerol... Motrin... Naproxen... Oxycontin... Percocet... Tylenol... Ultram... or Vicodin... and your pain will simply disappear.
And that's fine - if you're not a fan of living.
While these drugs may be working right now to temporarily stop your pain... they are secretly working behind the scenes... tearing apart your stomach... turning your organs into mush... and causing your insides to become leaky faucets of BLOOD!
Don't believe me?
Here's proof.
Adverse drug reactions are the 4th leading cause of death in the United States... and even put your life at risk in the hospital... affecting more than 1 in 10 patients.
Only Heart Disease, Cancer, And Strokes Kill More Americans Every Year!
Here are just a handful of examples of commonly prescribed pain meds and their health-crushing effects...
Aspirin - Want to DOUBLE your chances of developing a perforated ulcer or gastrointestinal bleeding? Then start taking "1-a-day" Aspirin and kiss your organs goodbye!
Tylenol/Acetaminophen - Every year, more than 56,000 Americans will visit the emergency room due to acetaminophen overdoses. It’s the leading cause of calls to Poison Control Centers and by far the leading cause of acute liver failure, causing nearly 50% of all cases.
Vicodin, Lorcet, Norco, Percocet, Percodan, Hydrocodone, Oxycodone - Over 16,000 people per year DIE from opiate overdoses. These highly addictive pain meds prescribed by pill-happy doctors cause liver failure, severe constipation leading to ruptured bowels, blocked bowels or sepsis... severe respiratory depression, confusion, coma, and complete cardiac arrest.
Advil, Aleve, ibuprofen, naproxen - These "everyday" pain meds carry a heavy burden. Much heavier than you would ever expect. Just check out these stats...
... The odds of dying from taking a nonsteroidal anti-inflammatory drug (NSAID) after just two months is around 1 in 1,200.
... Roughly 16,685 people die each year from NSAID related complications... stealing the life away from as many people as AIDS!
Celebrex - Feeling like playing a little Russian roulette with your heart and brain? Just take prescription Celebrex and you'll instantly triple your risk of heart attack or stroke!
While studying the drug’s potential as an anti-cancer drug, the National Cancer Institute discovered that...
"Those taking 400mg doses had 250% greater risk of dying from heart attack or stroke... and those taking the 800mg doses has 340% times the risk!"

If you want to play "prescription roulette... just take Celebrex and triple your risk of heart attack!
So the question is...
... WHY are these pain medications so harmful to your body?
... WHY do they put you at risk for so many other side effects?
... WHY have you been betrayed and lied to by the drug companies and doctors who prescribe you these potentially deadly pain meds?
Dirty Little Secret #2:
IF You're In Pain - PLEASE Don't Fall Victim To
The Greedy, Money-Hungry Drug Corporations Feeding You Lies!

These greedy drug corporations want one thing... YOUR MONEY... even if the drugs they're selling you are killing you!
Here's proof.
In 2006, revenues of prescription drugs topped $643 BILLION (yes, with a B) dollars...
Wanna know WHERE that excess of money is going?
It's going to places like...
-
The pocket of Jeffrey Kindler - Jeffrey Kindler is the chairman of the company who creates the popular prescription painkiller "Celebrex". The same one that TRIPLES your risk of heart attack or stroke. To give you a taste of how "ethical" the company is... they pleaded guilty to the largest health care fraud in U.S. history in the year 2009. To make matters worse - this was the 4th time they were charged with that same crime in the past 10 years!
So how much of YOUR money goes into his pocket every year? Well... way back in the year 2007... his salary was a whopping $12,600,000.
Yes... $12.6 MILLION dollars.
That's 1.5 million per MONTH... $242,307.69 per WEEK... $34,615 per DAY!
Now listen, I'm not against making money. Everybody wants to make more money, however, I believe in making money by helping people... not making them sick and even killing them!
-
Your Doctors And Physicians - Baffled at the fact that every time you go to the doctors, you're quickly pushed out the door with "yet another" prescription?
Here's why.
Sales representatives for these drug companies shower your doctors with free samples and office supplies in order to persuade them to prescribe you the medication they're selling.
Some companies offer so-called "educational opportunities" for physicians to continue the medical training necessary to renew their medical licenses. Free dinners, paid trips, and speech slots at symposiums can all influence your physician’s decision to prescribe one prescription over another.
-
Corrupt Government Officials - Ever wonder why your wallet gets lighter every time you even THINK about your prescriptions?
It's because no-good drug companies have a team of 625 lobbyists lining the pockets of our own Congress so they don't pass any "price limitations" on the pain meds and other prescription medicines you currently take.
In a recent election, drug companies spent more than ANY other industry in the world to sway the politicians in their favor... influencing politicians to pass bills in their favor with a whopping $134 MILLION dollars.
Can you believe this madness?
It's no wonder so many thousands of people get sent to the hospital, develop life-altering diseases and problems within their bodies... and even DIE from taking NSAIDs and prescription pain meds.
All the drug companies care about is the money... NOT you!
But that's not the biggest problem.
The biggest problem is that... while the pain meds you're currently taking are slowly eating away at your vital organs like a deadly virus... they're literally INCREASING the amount of pain your body is in.
Let me explain...
Dirty Little Secret #3:
The Longer You Use Traditional Pain Meds...
The LESS Effective They Become!
Many of today's most popular pain medications aspirin, ibuprofen, Advil, Aleve, Celebrex and Motrin - are designed to fight inflammation. Others, like Tylenol and Panadol... block incoming pain signals.
But none of them target what may actually be at the root of your pain: fibrin.
Fibrin is a natural substance in your body which helps in wound healing. It's also what scar tissue and scabs are made of.
Here's how it works: Your body senses an injury - it can be as minor as a small bump, scratch or even just sudden heat or coldness - and it reacts by:
#1: Sending white blood cells to the irritation or injury to fight infection, causing inflammation and swelling.
#2: Releasing fibrin to essentially "seal off the site" with a strong protective mesh.
NORMALLY... the site is healed, the fibrin breaks down, the inflammation subsides, and everything goes back to normal.
But many times...
This Process Goes Haywire... Causing Spiderwebs Of Scar Tissue To Pump Through Your Body!
Sometimes, our bodies fail to "call off" the fibrin. So like a button that's stuck in the "ON" position, fibrin continues to build up around the site, then harden - causing further inflammation, and often excruciating pain that just won't go away.
Plus, research studies show that if you're over 50, once inflammation is triggered, it's much less likely to go back down.
Meanwhile, you keep tossing all these painkillers at the fibrin. But it's like trying to break into a walnut shell with a water gun. In this case, all the painkillers in the world won't work if they can't get through the fibrin!
In other words, the longer you ignore the REAL cause of the problem... the fibrin... the longer you'll stay in pain and the less effective traditional pain meds will be in managing your pain.
But don't worry because...
We've Discovered A Way To BUST Through The Fibrin, Lower Inflammation And Free Your Body From The Pain!
It starts with a man named Dr. Max Wolf.

He was a medical doctor at the prestigious Columbia University for over 30 years and was doing research on why your pain seems to increase as you age.
In particular he was looking for the differences in why young people often experience faster healing times and less overall pain than older folks.
Here's what he found.
He discovered that when the body was reacting to pain, the bodies of younger adults responded by flooding the painful area with something called "proteolytic enzymes".
But for some reason... the older folks he studied showed DRAMATICALLY reduced levels of proteolytic enzymes and therefore felt more pain.
Excited by his amazing discovery, he narrowed it down even further and discovered that beginning around age 27 the amount of proteolytic enzymes in your body begins to diminish.
Bottom Line: If you're over 27 years old...
THIS may be the reason you're still in pain.
Could Proteolytic Enzymes Be Acting As
The Shut-Off Switch For Pain?
Dr. Wolf's discovery that younger adults have dramatically higher levels of proteolytic enzymes in their bodies launched a wave of controversy and excitement into the scientific community.
And what scientists found was nothing short of amazing...
In 1972 they discovered that proteolytic enzymes are the first line of defence against pain and inflammation!
Back pain... Sciatica... Chronic Fatigue... Fibromyalgia... Joint Pain... High Cholesterol... Arthritis... Cancer... Heart Disease... High Blood Pressure... Obesity... Depression... or Colitis!
Did you know that...
... A newborn baby’s enzyme levels are 100 TIMES HIGHER than that of an elderly persons?!
... A 20-year-old has double the enzyme levels of a 70-year-old...
... Grey hair can be caused by a lack of the enzyme tyrosinase.
... Blood clotting disorders are often associated from a lack of the enzyme plasmin.
This is just the tip of the iceberg. There are thousands more enzymes that are responsible for tens-of-thousands of daily, protective reactions in your body every day.
That’s why it’s critical that you replenish your enzyme stores!
Amazingly, they operate on a “lock-and-key” basis which means they can recognize good prostaglandins from bad prostaglandins.
And when their teeth fit into a ‘bad’ prostaglandin that's already run its course and has no more use... they dispose of it to let the GOOD prostaglandin come in and get rid of the pain.
They also discovered that...
- Proteolytic enzymes are completely safe - In 1993 scientists discovered that proteolytic enzymes have no known lethal dose. Compare that to NSAIDS which put hundreds of thousands of people a year in the hospital and, in some cases can cause death!
- A 1982 study showed proteolytic enzymes “eat” fibrin and other scar tissue – this may explain why as you age your wounds heal with thicker, weaker, less pliable and more visible scars. It's because you don't have enough proteolytic enzymes to "eat up" that scar tissue!
- They cleanse toxins from the blood – believe it or not, cells and organs dispose of toxins through your blood. The proteolytic enzymes soak up these toxins and get rid of them!
- Your blood becomes thick from too much scar tissue which turns it to sludge, causing high blood pressure, lack of circulation, clots and many other problems. Enzymes reduce the scar tissue... allowing your blood to flow smoother and helping your body cleanse toxins.
- Enzymes fight viruses and improve your immune system - Proteolytic enzymes recognize viruses through their special lock-and-key mechanism just like they recognize the prostaglandins, so you get sick less often!
Proteolytic enzymes have even been used in Europe since 1974 to treat cold sores and other viral conditions... and that's even before people knew what they were called!
Proteolytic Enzymes Have Been PROVEN To Work By Some Of The Top Research Centers In The World!

- Another study done by the Institute of Cancer Research in Vienna found that "a combination of proteolytic enzymes and herbs were effective in treating rheumatoid arthritis and reducing TGF-b which is known to be present in the blood when there is cancer."
- In a study done by Dr. Nicholas Gonzales, which was published in the medical journal, Nutrition and Cancer, Dr. Gonzales compared systemic enzyme therapy against a new cancer drug, Gemcitabine. He found that 5 out of 11 patients treated with systemic enzymes lived two years or longer and not a single patient of the 129 treated with the drug lived more than 19 months!
- A study published in the Journal of Medicine, Science, Sports and Exercise found that “proteolytic enzymes have therapeutic effects in the treatment of inflammation and soft tissue injuries.”

Keep in mind that drug companies can't patent natural substances like vitamins, minerals and, yes, proteolytic enzymes.
(They want to patent their pain meds so competitors can't steal them)
Why do you think proteolytic enzymes aren't included in NSAIDs and prescription pain meds? Because if the drug companies added them into their formula... competitors would steal them and they'd lose money!
Once again... when it comes to drug companies... they care about the MONEY... not you.
Of course, they can try to come close and mimic their actions. Which is what they tried to do with the popular NSAIDS. But as you’ve discovered, sometimes "mimicking" actually makes things worse.
So the question now becomes... how do you get them into your body?
The answer is simple...
Food Alone CAN'T Boost Your Enzyme Levels High Enough To Relieve Your Pain!
So far, you’ve discovered 3 scientific facts...
FACT #1: Both prostaglandins and fibrin – two normal protective and healing processes – are occurring in your body right now. Unfortunately, if they continue unchecked they can cause more harm than good.
FACT #2: Proteolytic enzymes have been proven to not only control prostaglandins and scar tissue, but even reverse some of the damage they’ve done. Better yet, they're also 100% safe.
FACT #3: Unfortunately, your production of proteolytic enzymes decreased dramatically around the age of 27. And with the poor nutrition content of most foods today it's becoming nearly impossible to replenish those stores through food alone.
Which leaves you with a decision to make.
... Do you want to take the risk of dying 5... 10... 15 years early?
... Do you want to continue wasting your money on expensive and dangerous prescription pain meds that are literally eating away at your organs like a cancerous Pac Man?
... Or maybe you're ready to try something NEW... something 100% safe... something all-natural which HEALS your body as it soothes away your pain?
If so, I’d like to introduce you to...
An All-Natural, 100% Safe, Pain-Relieving Supplement Which HEALS Your Body As It Soothes Away Your Pain!
It’s called "Heal-n-Soothe®" and it not only gives you the healing power of proteolytic enzymes in a convenient capsule but it also goes where no other proteolytic supplement has or will with...
- Today’s Most Powerful Proteolytic Enzyme Combination: We've combined the incredible systemic enzymes Protease AM, Protease 6.0 and Alkaline Protease... Bromelain and Papain to give you the most powerful pain-fighting effects!
- No Label ‘Sleight of Hand’: Most supplements don't list the exact concentrations on their labels... because they want to hide the fact that they're RIPPING YOU OFF! As you'll see below... you get to see the entire label... with ALL ingredients and ALL concentrations of each ingredient - because we have nothing to hide.
- The Only Truly All-Natural Proteolytic Enzyme Formulation - Available with no dangerous fillers or preservatives! This pain-reducing miracle was made for the pain sufferer who wants to get rid of their pain without adding mystery chemicals into their body.
“This is one of the best formulated products I’ve seen.. I give it my strongest recommendation”
- Dr. Robert Thompson, MD
“This Stuff Is God In A Bottle”
- Greg Moormann
“It Has Given Me My Life Back”
- Theresa Klein
“The KEY that made me well again”
- Alex Mitchell
“I have never felt better in my life... I stopped taking 2 prescription drugs because of this”
- Steve Olson
“This stuff saved my life”
- Ruth Novoa
“I’m pain free... I don’t get sore like I used to...”
- Lois Purser
“I love Heal-n-Soothe... I’m able to walk around and do the work I need to do”
- Kate Method
“By About 4 Month I Was Totally Pain Free”
- Lorie Stricker
“My pain level was a 10”
- Kalen Vavla
But even beyond the healing power of proteolytic enzymes, Heal-n-Soothe® has added...
12 Additional Proven Anti-Inflammatories
Straight From Mother Nature Herself...
These anti-inflammatories combine with proteolytic enzymes to give your body a powerful and proven one-two punch against inflammation and pain.
This powerful inflammation fighting formula includes...
Proteolytic Enzymes
Research indicates these enzymes work throughout your entire body to help it fight inflammation... dissolve scar tissue... cleanse and thin the blood... plus even boost cardiovascular, respiratory and immune function.
In other words, proteolytic enzymes are the final line of defense against disease, illnesses, pain and everything else that happens inside your body.
And unfortunately with the nutrient-deficient food we're eating today, the vast majority of adults today have dangerously low levels of these enzymes!
The easiest and most affordable way to get them in your body is by taking Heal-n-Soothe® on a regular basis.
Turmeric Extract

Turmeric has the unique ability of using its antioxidant powers to seek out and destroy free radicals in the body which contribute to pain and swelling.
A recent study done at the prestigious Sloan-Kettering Cancer Research Center in New York.
In this study they found that Turmeric was more safe and precise than aspirin in stopping inflammation by shutting down the COX2 enzyme responsible for pain.
And Turmeric caused none of aspirin's typical gastrointestinal irritation!
Devil's Claw

Numerous studies have proven it's effectiveness in reducing pain and inflammation.
In fact, one study found that Devil's Claw was as effective in treating arthritis pain as a commonly prescribed anti-inflammatory drug... which is so strong it's even used to treat pain in horses!
There have also been several studies on humans which show that Devil's Claw is as effective as COX-2 inhibitors... without the deadly side effects!
A series of studies completed in Germany found that the main ingredient in Devil's Claw was indistinguishable from Vioxx in the treatment of chronic low back pain!
Mojave Yucca (root)

The Mojave Yucca root has more benefits than you could shake a stick at!
These improvements in your health include migraine relief... decreased blood pressure, cholesterol and triglycerides...better digestion... reduction of muscle spasms... improved blood circulation... treating various skin conditions... healing wounds... and it even promotes shiny and healthy hair.
Rutin
Found naturally in a variety of plants and fruits, this flavonoid has been shown to have a strong anti-inflammatory effect due to it's powerful anti-oxidant activity.
Reducing your inflammation leads to changes throughout your entire body.
Changes such as ... a reduction of pain... increased circulation and dozens of other health benefits which ramp up your energy!
Bromelain

This special natural compound which is extracted from pineapple contains several proteolytic enzymes that have been shown to short-circuit multiple pain pathways in the body.
It has been studied extensively since it's discovery in 1957 and hundreds of studies have shown it to reduce inflammation, reduce and prevent swelling and remove waste and toxins from the blood.
For example, in one study, 77 patients taking Bromelain experienced significant reduction in pain and swelling.
Papain
Papain is unique in that it's been shown to actually attack tumor cells and boost the immune system!

It also contains a wide range of proteolytic enzymes and works by breaking down proteins.
In studies it has been shown to be effective in the treatment of numerous conditions such as diabetes, herpes, cancer and digestion issues like bloating and chronic indigestion.
It's an enzyme very few people have enough of, yet if they did, would reduce the frequency of a lot of common diseases and disorders!
Boswellia Extract

One of the most widely used herbs in Indian medicine, it has been used for centuries by traditional Indian healers to reduce pain and inflammation.
Hundreds of studies have been done proving it's effectiveness and in addition to being a powerful and safe, natural anti-inflammatory, Boswellia has also been shown to support healthy blood circulation.
In a randomized, double-blind, placebo controlled study, 30 patients received 333mg, 3x a day of Boswellia.
The group who received the Boswellia had a significant reduction in pain and swelling and experienced an increase in joint mobility and flexibility as compared to the placebo group.
Ginger Extract

Used for over 2,500 years in Asia, Ginger has been used to treat nausea and to reduce pain and inflammation.
It works by decreasing the amount of prostaglandins, which are what cause you to feel pain. In other words, it does what NSAIDs and prescription pain meds do, but in the way nature intended it to do so... instead of forcing it and causing side effects!
Citrus Bioflavonoids
Citrus Bioflavonoids aid in the absorption of vitamins and act as important antioxidants.
They also inhibit collagenase and elastase, the enzymes responsible for the breakdown of connective tissue.
That way your joints and ligaments stay as healthy as when you were a teenager!
In addition to protecting connective tissues, they also protect against free radical damage.
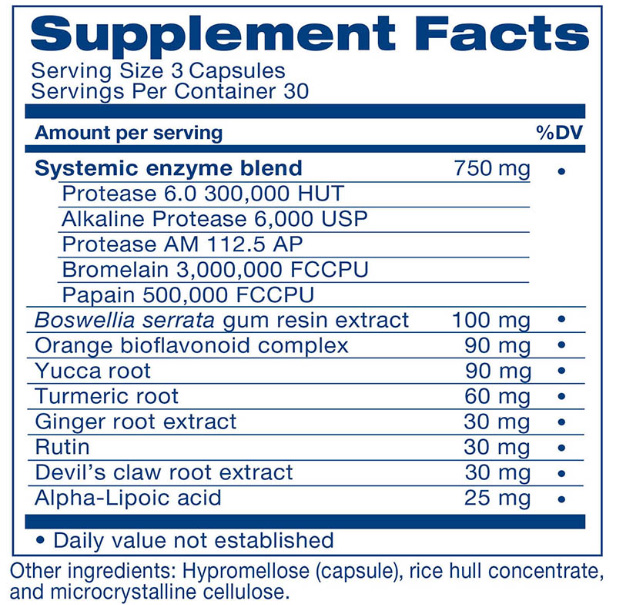
As you can see, we're not hiding anything. Here is the EXACT list of ingredients AND concentrations!
On their own, each of these ingredients have been proven through both clinical studies and thousands of years of use to be safe and effective.
But together, they work synergistically to quickly reduce inflammation and pain.
Heal-n-Soothe® not only has the most powerful combination of natural anti-inflammatory and pain relieving ingredients, it also has the strongest and highest potency of any proteolytic enzyme formulation available!
And, unlike most supplement manufacturers who will call it a "proprietary blend" and don't say how much of each is included, we show you exactly how much of each ingredient is in the product.
Not only can you not find a more effective and powerful supplement, but you can't even purchase an enzyme formula like this at your local drug store.
We even had a customer call in recently to say he asked his local pharmacist if they had any systemic enzymes and they didn't even know what he was talking about... scary!
Speaking of customers... right now you're probably wondering whether or not Heal-n-Soothe® really works. And it's a great question. I'd be asking the same question if I were in your shoes.
I've already shown you PROOF that each and every ingredient in Heal-n-Soothe® was specifically designed to eliminate your pain and HEAL the damage done by NSAIDs and prescription pain meds.
But that's not enough.
Let me reveal a few of our recent success stories from people just like you who took a chance and tried Heal-n-Soothe® and finally found lasting relief without the harmful side effects!
Even MORE Success Stories...
95% Pain-Free After First Bottle of Heal-n-Soothe®!
I took the first bottle of Heal-n-Soothe®, and I am about 95 percent pain free!
-- Ina Mae McBride
Claudville Va
Five Days Later The Pain Was Gone And Hasn’t Come Back!
"As a scientist I was somewhat skeptical of the Heal and Soothe product, given that it was one of many natural pain relieving products available on the web. However, after about 12 months of lower back pain I thought I have nothing to lose. The bottle arrived and I started taking them. At about that time, I had the back pain diagnosed as a slightly herniated disc and so I thought “great, now I have a lifetime of pain ahead of me". However, the pain did subside and disappear... but, being a scientist, I didn’t know if it was the tablets or perhaps the psychosomatic effect of the diagnosis that helped relieve the pain. I still kept taking the Heal and Soothe, albeit irregularly, and didn’t really have much trouble. Then without realizing it, I ran out of tablets and thought oh well, lets see how things go. A while later my back started to hurt again and over a period of a week it was back to as bad as it was before. I couldn’t work out what I had done to aggravate it. I suddenly realized I hadn’t been taking the tablets and, as another bottle had recently arrived, I started taking them again. Five days later the pain was gone and hasn’t come back! I have also had long term muscle knots in my right shoulder blade because of poor computer posture. Guess what! That has gone too. This stuff works!"
-- Dr. Mike Jones
Australia

"Mom Of 4 Ditches Her Liver-Damaging Celebrex For Heal-n-Soothe® And LOVES The Results!"
I've had orthopedic problems since I was 7 years old. I figured up that over the last 31 years (I'm 38 now), I've had a total of 14 surgeries---6 of them knee surgeries and 1 foot surgery---plus a broken pelvis and sprained SI joint from a past car accident.
After the foot surgery 10 years ago, the doctor decided I would benefit from taking Celebrex.
Recently, after being diagnosed with severe arthritis in one knee and moderate arthritis in my foot, the doctor doubled my dosage of Celebrex. I couldn't discern any improvement on the new dosage. Also, I began getting concerned about the possibility of the prolonged usage of this drug causing liver damage.
I have now been taking Heal-n-Soothe® for 30 days, and I feel the same as I did while taking the double dosage of Celebrex.
I have hope that after more time on Heal-n-Soothe®, I will feel even better---and I like that right now I'm getting at least the same, if not yet better, anti-inflammatory effect from natural enzymes, rather than from a chemical that could potentially harm my liver.
Thanks so much!
-- Kaye Whitlock
stay-at-home Wife & Mom to 4 kids
"More relief from Heal-n-Soothe® than I got from Naproxen!"
I am an RN working 12 hr night shifts and I have fibromyalgia. I take Lyrica 3 times a day. I received the bottle of Heal-n-Soothe® as I was leaving for work tonight, was aching all over, I was going to take some naproxen, but on opening the envelope, I decided to take Heal-n-Soothe® instead.
It's approx 4 hrs later and I got more relief from Heal-n-Soothe® than I often get from naproxen! Not bad for the first dose! I'm hopeful.
Thank you!
-- Carol
"I can't imagine life without Heal-n-Soothe®!"
"I reallly think the Hean-Soothe® have been doing a great job on me! I have just started my 2nd month of using them and for right now I can't imagine life without them! I do feel better. Thanks for a great product."
-- Sandy Underwood
"WOW! Huge difference! I stopped taking painkillers!"

"All I can say is a big WOW and thank you. I have only been taking them for 4 or 5 days, but have noticed an huge difference. I stopped taking my strong painkillers, Co Codemal (the strongest) and Dihydrocodine, which I was taking every day and the full limit allowed in a day. I feel "almost" normal again."
From a very very grateful,
-- Angela Jaggs (England, UK)
“All I Can Say is WOW... Send Me Some More!”
Great product you have in Heal-n-Soothe®. I have been taking it for about 4 months now and all I can say is, WOW, what a difference!
I wish I had known about Enzyme therapy sooner. I noticed a difference right away and now when I hit it hard on the tennis court or in any other activity I increase the dosage for a few days after and my recovery is almost instant.
All I can say is thank you for the help and support and send me some more!
-- Nicholas Batt
Dallas, TX
"Thank You!"
Dear Jesse

"Thank You for all the Emails you have been sending me since I enrolled in the "Lose the Back Pain® System"
I also purchased the Heal-n-Soothe® Capsules at the very Welcome Price!
I stopped taking all NSAIDS Immediately (which I have been taking for five years) and started taking the Heal-n-Soothe®® Capsules - To my delight about half the burning sensations in the front and sides of my shins disappeared by the second day of use.
The awful debilitating Cramps in my Calves and Feet ceased almost altogether - Leaving me now to contend with ' Only ' Sciatica in my right buttock and leg - numbness in both my feet and toes and pains in the soles of my feet and heels, like standing on Golf Balls - which in itself is a Great improvement - So I will continue with a further course of the Heal-n-Soothe® Capsules :-)
We have enjoyed Every moment of the Video and Audio and the Emails and also the Further Links - Our heads are Spinning - with glee at the prospect of Help may yet be at hand !!
Best Wishes to You All
-- Chris and John
"My pain and inflammation went down in just 2-days!"
"I have been taking Heal-n-Soothe®, for 3 weeks now. I must say at first I was very skeptical, I was thinking "How can this really help if my medication the doctor gave me does not work??!" But sometimes you reach a point or a day where the pain is so bad, that you will try anything, any price, just anything that might have a slight chance of getting rid of your pain...So I did.
And I was pleasantly surprised, after about 2-3 days on the activation dose, I noticed reduced inflammation, and my pain in my leg reduced. At first I was thinking "Maybe its just the nice weather we have here in the U.K at the moment?" : )
I realized that it must be the Heal-n-Soothe®, because I missed one days dosage and the next day I felt really bad : ( It took 2 days after to get things back on track, and I make sure I do not forget them now. It means that I am able to stretch further and more often as the other post stated, and walk more. I'm a believer that you "Can't put a price on your health"."
-- Dan Monahan, England
"Prior To Heal-n-Soothe I Would Always Be In Pain"

"I want to thank you for offering Heal-n-Soothe to me! The first time I tried the supplement I didn't get any relief, but I just didn't give it enough time. Prior to Heal-n-Soothe, I would be in pain bowling, mowing lawn, picking weeds, etc. and now it is all better, as long as I don't push it. Thank you HEALTHY BACK INSTITUTE!""
-- Linda Hodgeman, Bullhead City AZ
"I can walk to the store again without pain!"
Jesse,
I tried the Heal-n-Soothe® free bottle that I received. You were right! It worked really good and I do feel much better. My back pain is much improved and since taking the Heal-n-Soothe® I can walk the 2 blocks to the grocery store, spend an hour gathering groceries, then walk back home with my cart of groceries. I don't even have to sit down or lie down when I get home and my back is not hurting. Not only that, but I seem to have more energy and generally feel better. Heal-n-Soothe® is great!
I really believe that if I continue with the Lose the Back Pain® exercises and keep working on my weight problem, I will soon not have to rely on pills of any kind. I am so glad I found and purchased your Lose the Back Pain® System and Heal-n-Soothe®. It has exceeded my expectations. I am improving rather slowly, but I AM STEADILY IMPROVING, thanks to you guys and the rest of your group.
Sincerely,
-- Barbara Adams
San Diego California area
"Thank you!!! Heal-n-Soothe® gave me a new
PAIN FREE life!"
Hey Jesse:
Just a note to tell you how much your Heal-n-Soothe® has helped me in just a week. It is working very well. I have a crooked spine, and sciatic nerve problems. Your product has given me a whole new life of PAIN FREE. I can actually golf now. Please keep sending every month. It has made a big difference in my life. Thank You... Thank you !!!!!
-- Sandra Marginet, Michigan
"My back-pain subsided to almost nothing and I don't have to 'play through the pain' anymore! Thank you so much!"

"Within the first week...
Recurring back pain from previous soccer seasons affected me again in August when I began my Fall soccer season. At first I was playing through the pain and then I started taking Heal-n-Soothe®. Within the first week I noticed a significant decrease in the amount of pain I felt.
I have been taking them now for nearly two months and over the course of these two months my back pain has subsided to almost nothing and I have been able to continue playing soccer all season. I truly believe that Heal-n-Soothe® has enabled me to get to where I am today which is playing pain-free.
Thanks, That is my story and thank you so much for your help!"
-- Lisa Brown
"WOW! What a difference!"

"I just wanted to tell you guys at the Healthy Back Institute® what a great product you have in this Heal-n-Soothe®. I have been taking it for about 4 months now and all I can say is, WOW, what a difference!
I wish I had known about Enzyme therapy sooner. I noticed a difference right away and now when I hit it hard on the tennis court or in any other activity I increase the dosage for a few days after and my recovery is almost instant.
All I can say is thank you for the help and support and send me some more!"
-- Nicholas Batt
"This stuff ROCKS! I felt improvement the first day! It eliminates aches and pains!"

"As a serious student of martial arts, my body has taken a beating and no matter how good you are about taking care of yourself, we all have aches and pains. Well, I have NEVER experienced anything that works as well as Heal-n-Soothe®... this stuff rocks!
I felt improvement the very first day and not only does it eliminate aches and pains, but my flexibility has also increased quite a bit. Now I take it after every hard training session to make sure I don't wake up sore, stiff and achy the next morning. If you have any aches and pains I suggest you give this stuff a shot!"
-- Otis Berry
Germantown, Maryland
-- Chief Krav Maga Instructor and One of Only 30 Krav Maga Black Belts in The United States
"Within 3 days of taking the capsules, I could not believe my good fortune..."

After reading your "free" book, I decided to order the "Heal-n-Soothe®" - because I had tried everything else. Within three days of taking the capsules, I could not believe my good fortune.
Even the Director of the Bay State Medical Group Pain Management Center could not believe the change.
As a graduate school professor, I must be on my feet in front of classes on a regular basis. "Heal-n-Soothe®" has been an answer to my prayers. Keep up the good work!
-- James Mundy
Graduate school professor
East Longmeadow, MA
As you can see, Heal-n-Soothe® WORKS.
There's no doubting it. All of these people tried it out, got the relief they've been searching for... and wanted to share their stories with you.
Those are just a small handful of the hundreds of reviews and success stories we've gotten over the past few years. Putting all of them on a single page would turn it into a small book!
Now you're probably wondering...
So How Much Does Fast,
100% Safe Pain Relief Cost?
"As a pharmacist I've seen it all. Heal-n-Soothe® is as effective as prescription anti-inflammatories, but
MUCH SAFER."
"As a pharmacist with a keen interest in natural supplements I can safely say I've seen it all. But for patients with pain - who are looking for an answer outside of dangerous prescription medications - I have only a handful of recommendations. Heal-n-Soothe® is on that short list.
It's not only as effective as prescription anti-inflammatories, but is much safer. And unlike other supplements on the market, Heal-n-Soothe® really is free of any fillers or colorants. It truly is all-natural.
The point is this: if you're sitting on the fence about other options to treat your pain you owe it to yourself to give Heal-n-Soothe® a fair shot. You'll be happy you did."

- Dr. Curtis Alexander
Registered Pharmacist
Doctor of Pharmacy, Montana
Does your health really "cost" you anything?
Think about it.
Your health is the single most valuable asset you have.
What would happen to your family if you continued taking NSAIDs and prescription pain meds and landed up in the hospital... your insides bleeding... like hundreds of thousands of people do every single year?
Besides the emotional torment your family (and you) would go through, it would be financially devastating to you. And even if you have a great boss who understood - how many precious years would you be shaving off your life?
What if that ONE single mistake - continuing to take traditional pain meds - ended up cutting your life short by a few years and stripping away the opportunity to watch your children get married... or graduate college... or see the birth of a new family member... or leave your spouse widowed?
THAT would be a costly mistake.
The easy solution is to simply not make that mistake. Instead, you can invest as little as $1.38 per day for a 30 day supply of Heal-n-Soothe® when you buy 6 bottles.
So tell me... if you could invest as little as $1.38 per day and receive not only a reducing or elimination in your pain... but also boost your immune system... cleanse the toxins from your body... get increased circulation, enhanced mobility and dozens more benefits...
... all WITHOUT risking the health of your body's most important vital organs such as your liver and kidneys...
... shouldn't that be a no-brainer for you?
I sure hope so!
And just in case for some reason it's not, I'd like to do something you'll NEVER find any NSAID or prescription pain medication do for you.
I'd like to GUARANTEE that Heal-n-Soothe® does everything I claim above.
Heal-n-Soothe® Is GUARANTEED To Work For You Or We'll Buy It Back!
We are so confident that Heal-n-Soothe® will reduce your pain and have you feeling better that we are willing to refund your money if it doesn't.
That's right... you can try it and all the risk is on us.
My Personal 90-Day Risk-Free...
100% Pain-Free Guarantee

We're so confident in Heal-n-Soothe® that we want you to try it out completely risk-free. Test it out and see if it works for you.
For single bottle orders:
If you don’t feel significant improvement after trying Heal-n-Soothe for at least 2 weeks, simply return the used bottle and we’ll gladly refund your purchase price (excluding shipping).
For multi bottle orders:
Return all unopened bottles and we will refund a prorated amount based on the number of unopened bottles you have returned, (excluding shipping).

NOTE: If you request a refund within the 90-Day Money Back Guarantee period, you need to return ALL Unopened Bottles and we will refund a prorated amount based on the number of unopened bottles you have returned, Less Shipping.
5 Great Reasons To Test-Drive
"Heal-n-Soothe®" Today
It HEALS Your Problem Instead Of Just Covering It Up - Unlike traditional pain medications which simply cover up your pain like a Band-Aid... Heal-n-Soothe® actually HEALS the underlying condition causing your pain. That means you'll feel quick pain relief in the short-term... and permanent healing in the long-term!
No Side Effects - Unlike traditional paid medications (NSAIDS)... when you order Heal-n-Soothe® today you'll never again have to worry about side effects such as internal bleeding, risk of liver failure or even dying! Heal-n-Soothe® is made with 100% natural ingredients and is as safe to take as your daily multivitamin. In fact... we recommend you take Heal-n-Soothe® WITH your multivitamin!
NO RISK! We've Put ALL The Risk On OUR Shoulders - Listen, I know you're in pain and you want to get rid of that nasty pain. Heal-n-Soothe® can help, but I'm sure you're still skeptical. That’s why we’re offering you our 90-Day Money Back Guarantee if for some reason this doesn’t relieve your pain. Either we deliver on our promise... or you won’t pay a penny for the bottle (only shipping)... we can’t make a better deal than that for you!
Pay As Little As $1.38 Per Day - Take a look at the chart below and you'll see that if you take us up on our "best deal"... you can be paying as little as $1.38 per day. Is HEALING pain relief worth $1.38 per day to you?
Feel ALIVE Again! - Journey back to the days of your childhood when aches and pains were only a rarity and you could do what you wanted, when you wanted without worrying about aches and pains getting in your way. With Heal-n-Soothe® that becomes a reality again as the unique formula increases those same chemicals responsible for healing and pain-relief that you had as a kid but started losing after age 27.

How Much Heal-n-Soothe®
Should You Take?
People experience pain differently… Some days you’re in more pain than others.
It’s not uncommon for people, who are in severe pain or who have had surgery in the past to take 12 or more capsules of Heal-n-Soothe a day.
By taking more, you give your body a better chance of clearing away DECADES of sticky buildup of scar tissue. And you never have to worry about taking too much. Because, Heal-n-Soothe is safe and non-habit forming.
Use your best judgement, to find what dosage works best for you.

Proven Anti-InflammatoriesStraight From Mother Nature Herself...



Still Have Questions?
Heal-n-Soothe® Frequently Asked Questions
A: Heal-n-Soothe® should be taken once per day on an empty stomach, 30 minutes before or 60 minutes after meals for best results. Some people find that a single dose before bed works best.
Begin by taking 3 capsules once per day. If you do not feel a reduction in pain at that dosage, experiment with a higher dosage by gradually increasing the dosage by 1-2 capsules per day until you find what works best for you.
A: Proteolytic enzymes have an excellent safety record, with no significant side effects reported. With any supplement, however, there is always the risk of developing an allergy to one or more ingredients. If this happens, you should discontinue use. *
A: NO, Heal-n-Soothe® is 100% natural and contains NO animal derivatives. *
A: Individuals taking any medication should consult physician prior to taking Heal-n-Soothe®, it is recommended that you wait at least 60 minutes after taking Heal-n-Soothe® before taking any medications. *
A: Persons who suffer from medical conditions or who are taking medications should consult their physician prior to taking this product. This product may thin the blood and may not be appropriate for all persons. Do not take this product if you know or suspect that you are allergic to pineapple, papaya, or any ingredients in this product or suspect that you have an ulcer. As with all dietary supplements, those who are pregnant or nursing should consult their physician prior to taking this product.*
Individuals taking any prescription blood thinners like but not limited to (ie. Coumadin, Heparin, Plavix, Xeralta, Eliquis)
Anyone who will be having surgery in less than two weeks
Individuals with known ulcers of the stomach
Pregnant or lactating women
Individuals currently taking antibiotics
Individuals with an allergic reaction to pineapples or papayas
Individuals under the age of 18
A: Heal-n-Soothe® can and should be taken daily along with your multiVitamin for as long as you want to keep inflammation in check and continued support for soft tissue recovery, improved joint function and maintaining a healthy immune response as well as support to cardiovascular and respiratory function. *
A: It is possible that you may experience the following effects on the body.
For individuals with sinus issues, you may experience some drainage for a short time as the mucus thins and is eliminated from the body. *
For individuals with digestive issues, you may experience some gas or loosing of your stool as undigested matter is broken down and eliminated. *
For individuals with borderline high blood pressure, as fibrin is eliminated from the body, there will be less resistance on the blood and thus your blood pressure could come down. *
For women with Uterine Fibroids, you may experience some vaginal discharge as the Fibroid is broken down and is eliminated. *

Outside US: 240-780-5977

USA: 866-843-4319
International: 240-780-5977
Now That You Know That You're Playing Prescription Roulette Every Time You Pop A Painkiller... What Will You Do About It?
In the past few minutes I've opened up your eyes to a whole new world where science has finally broken through and given us what we consider to be the single most powerful 100% safe all-natural pain relieving formula ever created.
And...
... you've felt secure knowing it includes our full 90-day money back guarantee...
... you've read all the scientific PROOF showing the ingredients used in Heal-n-Soothe® are 100% safe, all-natural and promote healing as well as pain relief...
... AND you've met dozens of other pain sufferers - just like you - who have already experienced what it's like to soothe your pain in just days...
My question now is - what will YOU do now that you know all these incredible benefits are available to you for as little as $1.38 per day?
If you're smart, like I already know you are... click here to see which package is right for you. Or, if you still have any questions you can feel free to call us toll-free at 1-800-216-4908 right now.
My friend, pain is not a fun thing.
I hope you make the right decision to banish it forever from your life by trying out Heal-n-Soothe® today.
Remember... if you give it a try and don't think it's everything we claim, simply return your order within 90 days. You re automatically covered by our 90-Day Money Back Guarantee.
Best wishes,
Jesse Cannone

Co-Founder, The Healthy Back Institute®
P.S. I've done the best I could to try and HELP you. If you want to continue paying the billion-dollar drug companies for an early death, that's up to you. But if you love living, want to watch your kids and grandkids grow up, and would like to live pain-free... try out Heal-n-Soothe® today. You have nothing to lose!
P.P.S. Still have questions? Take a look at our FAQ to make sure Heal-n-Soothe® is right for you...
Clinical Studies Used In The Research and Development of Heal-n-Soothe®
1) Taussig SJ, Batkin S. Bromelain, the enzyme complex of pineapple (Ananas comosus) and its clinical application. An update. J Ethnopharmacol. 1988 Feb-Mar;22(2):191-203.
Source
Department of Food Science and Human Nutrition, School of Tropical Agriculture, University of Hawaii, Honolulu.
Abstract
After a short description of the uses of pineapple as folk medicine by the natives of the tropics, the more important new pharmaceutical applications of bromelain, reported between 1975 and 1978, are presented. Although the exact chemical structure of all active components of bromelain is not fully determined, this substance has shown distinct pharmacological promise. Its properties include: (1) interference with growth of malignant cells; (2) inhibition of platelet aggregation; (3) fibrinolytic activity; (4) anti-inflammatory action; (5) skin debridement properties. These biological functions of bromelain, a non-toxic compound, have therapeutic values in modulating: (a) tumor growth; (b) blood coagulation; (c) inflammatory changes; (d) debridement of third degree burns; (e) enhancement of absorption of drugs. The mechanism of action of bromelain affecting these varied biological effects relates in part to its modulation of the arachidonate cascade.<
2) Source
Emeruwa AC. Antibacterial substance from Carica papaya fruit extract. J Nat Prod. 1982 Mar-Apr;45(2):123-7.
Abstract
Ripe and unripe Carica papaya fruits (epicarp, endocarp, seeds and leaves) were extracted separately and purified. All the extracts except that of leaves produced very significant antibacterial activity on Staphylococcus aureus, Bacillus cereus, Escherichia coli, Pseudomonas aeruginosa and Shigella flexneri. The MIC of the substance was small (0.2-0.3 mg/ml) for gram-positive bacteria and large (1.5-4 mg/ml) for gram-negative bacteria. The substance was bactericidal and showed properties of a protein. Other proteins previously found in C. papaya did not show antibacterial activity.
3) Source
Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC Jr, Taubert K, Tracy RP, Vinicor F; Centers for Disease Control and Prevention; American Heart Association. A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003 Jan 28;107(3):499-511.
II. Evidence for Inflammation as a Key Pathogenetic Mechanism in Atherosclerosis
A role for inflammation has become well established over the past decade or more in theories describing the atherosclerotic disease process.4,5 From a pathological viewpoint, all stages, ie, initiation, growth, and complication of the atherosclerotic plaque,6,7 might be considered to be an inflammatory response to injury. The major injurious factors that promote atherogenesis—cigarette smoking, hypertension, atherogenic lipoproteins, and hyperglycemia—are well established. These risk factors give rise to a variety of noxious stimuli that elicit secretion of both leukocyte soluble adhesion molecules, which facilitate the attachment of monocytes to endothelial cells, and chemotactic factors, which encourage the monocytes' migration into the subintimal space. The transformation of monocytes into macrophages and the uptake of cholesterol lipoproteins are thought to initiate the fatty streak. Further injurious stimuli may continue the attraction and accumulation of macrophages, mast cells, and activated T cells within the growing atherosclerotic lesion. Oxidized low-density lipoproteins may be one of several factors that contribute to loss of smooth muscle cells through apoptosis in the atherosclerotic plaque cap, and secretion of metalloproteinases and other connective tissue enzymes by activated macrophages may break down collagen, weakening the cap and making it prone to rupture. This disruption of the atherosclerotic plaque then exposes the atheronecrotic core to arterial blood, which induces thrombosis. Thus, virtually every step in atherogenesis is believed to involve cytokines, other bioactive molecules, and cells that are characteristic of inflammation. http://circ.ahajournals.org/content/107/3/499.full
4) Leonard BE. Inflammation, depression and dementia: are they connected? Neurochem Res. 2007 Oct;32(10):1749-56.
Source
Department of Psychiatry and Neuropsychology, Brain and Behaviour Research Institute, University of Maastricht, Maastricht, The Netherlands. belucg@iol.ie
Abstract
Chronic inflammation is now considered to be central to the pathogenesis not only of such medical disorders as cardiovascular disease, multiple sclerosis, diabetes and cancer but also of major depression. If chronic inflammatory changes are a common feature of depression, this could predispose depressed patients to neurodegenerative changes in later life. Indeed there is now clinical evidence that depression is a common antecedent of Alzheimer's disease and may be an early manifestation of dementia before the cognitive declines becomes apparent. This review summarises the evidence that links chronic low grade inflammation with changes in brain structure that could precipitate neurodegenerative changes associated with Alzheimer's disease and other dementias. For example, neuronal loss is a common feature of major depression and dementia. It is hypothesised that the progress from depression to dementia could result from the activation of macrophages in the blood, and microglia in the brain, that release pro-inflammatory cytokines. Such cytokines stimulate a cascade of inflammatory changes (such as an increase in prostaglandin E2, nitric oxide in addition to more pro-inflammatory cytokines) and a hypersecretion of cortisol. The latter steroid inhibits protein synthesis thereby reducing the synthesis of neurotrophic factors and preventing reairto damages neuronal networks. In addition, neurotoxic end products of the tryptophan-kynurenine pathway, such as quinolinic acid, accumulate in astrocytes and neurons in both depression and dementia. Thus increased neurodegeneration, reduced neuroprotection and neuronal repair are common pathological features of major depression and dementia. Such changes may help to explain why major depression is a frequent prelude to dementia in later life.
5) Barzilay JI, Abraham L, Heckbert SR, Cushman M, Kuller LH, Resnick HE, Tracy RP. The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes. 2001 Oct;50(10):2384-9.
Source
Division of Endocrinology, Kaiser Permanente of Georgia, and the Division of Endocrinology, Emory University School of Medicine, Atlanta, Georgia 30084, USA. joshua.barzilay@kp.org
Abstract
Several studies suggest that inflammation plays a role in the pathogenesis of some glucose disorders in adults. We tested this hypothesis in a longitudinal cohort study of older individuals who had normal fasting glucose (FG) values at baseline. We compared the baseline levels of six inflammatory markers in participants who had developed glucose disorders at follow-up with those of participants whose FG remained normal at follow-up. Participants were members of the Cardiovascular Health Study, a prospective study of risk factors for cardiovascular disease in adults > or =65 years. All 5,888 participants had baseline testing, including FG and markers of inflammation: white blood cell and platelet counts and albumin, fibrinogen, C-reactive protein (CRP), and factor VIIIc levels. At 3-4 years of follow-up, 4,481 (84.5%) of those who were alive had FG levels retested. Participants who developed diabetes (n = 45) had higher median levels of CRP at baseline than those who remained normoglycemic. On multivariate analysis, those with elevated CRP levels (75th percentile [2.86 mg/l] vs. 25th percentile [0.82 mg/l]) were 2.03 times (95% confidence intervals, 1.44-2.86) more likely to have diabetes on follow-up. Adjustment for confounders and other inflammatory markers did not appreciably change this finding. There was no relationship between the development of diabetes and other markers of inflammation. Inflammation, as measured by CRP levels, is associated with the development of diabetes in the elderly. Understanding the role of inflammation in the pathogenesis of glucose disorders in this age-group may lead to better classification and treatment of glucose disorders among them.
6) Festa A, D'Agostino R Jr, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 2000 Jul 4;102(1):42-7.
Source
Department of Medicine, University of Texas Health Science Center at San Antonio, 78228-3900, USA. festa@magnet.at
Abstract
BACKGROUND:
Inflammation has been suggested as a risk factor for the development of atherosclerosis. Recently, some components of the insulin resistance syndrome (IRS) have been related to inflammatory markers. We hypothesized that insulin insensitivity, as directly measured, may be associated with inflammation in nondiabetic subjects.
METHODS AND RESULTS:
We studied the relation of C-reactive protein (CRP), fibrinogen, and white cell count to components of IRS in the nondiabetic population of the Insulin Resistance Atherosclerosis Study (IRAS) (n=1008; age, 40 to 69 years; 33% with impaired glucose tolerance), a multicenter, population-based study. None of the subjects had clinical coronary artery disease. Insulin sensitivity (S(I)) was measured by a frequently sampled intravenous glucose tolerance test, and CRP was measured by a highly sensitive competitive immunoassay. All 3 inflammatory markers were correlated with several components of the IRS. Strong associations were found between CRP and measures of body fat (body mass index, waist circumference), S(I), and fasting insulin and proinsulin (all correlation coefficients >0.3, P<0.0001). The associations were consistent among the 3 ethnic groups of the IRAS. There was a linear increase in CRP levels with an increase in the number of metabolic disorders. Body mass index, systolic blood pressure, and S(I) were related to CRP levels in a multivariate linear regression model.
CONCLUSIONS:
We suggest that chronic subclinical inflammation is part of IRS. CRP, a predictor of cardiovascular events in previous reports, was independently related to S(I). These findings suggest potential benefits of anti-inflammatory or insulin-sensitizing treatment strategies in healthy individuals with features of IRS.
INFLAMMATION IS ASSOCIATED WITH INSULIN RESISTANCE.
7) Walker JA, Cerny FJ, Cotter JR, Burton HW. Attenuation of contraction-induced skeletal muscle injury by bromelain. Med Sci Sports Exerc. 1992 Jan;24(1):20-5.
Source
Department of Physical Therapy/Exercise Science, State University of New York, Buffalo 14214.
Abstract
The proteolytic enzyme, bromelain, reportedly has therapeutic effects in the treatment of inflammation and soft tissue injuries. We tested the hypothesis that bromelain attenuates skeletal muscle injury induced by lengthening contractions. The left extensor digitorum longus (EDL) muscle of anesthetized hamsters was injured using a motorized foot pedal which repeatedly flexed/extended the foot through a range of 125 degrees. The EDL muscle was electrically stimulated for 400 ms during plantarflexion. Animals were assigned randomly to either a 0-d group (evaluated 3-h post-injury) or to untreated (UT) or bromelain-treated (T) groups, evaluated 3, 7, or 14 d post-injury. Following injury, T received 5 mg.kg-1 b.w. of bromelain, twice daily. Maximum isometric tetanic force (Po) was measured in vitro, then muscles were fixed, sectioned, and examined for evidence of fiber damage. The Po of injured muscles from T were higher than Po of injured muscles from UT at 3 (18.7 +/- 0.4 vs 16.5 +/- N.cm-2 and 14 d (20.5 +/- 0.6 vs 18.2 +/- 0.6 N.cm-2) (P less than 0.05), but not 7 d (19.5 +/-0.7 vs 17.7 +/- 0.8 N.cm-2). The Po of UT injured muscles were significantly lower than Po of contralateral control muscles at all time periods. Po of injured muscles from T were lower than Po from control muscles at 3 and 7 d (P less than 0.05), but not 14 d. The number of intact fibers of 3-d UT injured muscles was lower than the number of intact fibers in control muscles (P < 0.05). No difference in fiber number between controls and the 3-d treated group was observed. Thus, daily oral bromelain treatments of 10 mg +/- kg-1 attenuated the development of contraction-induced injury in hamster EDL muscles.
8) Walker AF, Bundy R, Hicks SM, Middleton RW. Bromelain reduces mild acute knee pain and improves well-being in a dose-dependent fashion in an open study of otherwise healthy adults. Phytomedicine. 2002 Dec;9(8):681-6.
Source
Hugh Sinclair Unit of Human Nutrition, The University of Reading, UK. a.f.walker@reading.ac.uk
Abstract
There is preliminary clinical evidence to support the contention that the anti-inflammatory and analgesic properties of bromelain help to reduce symptoms of osteo- and rheumatoid arthritis. However, there have been no controlled studies of its effects on joint health in healthy subjects who lack such diagnosis. The current study investigated the effects of bromelain on mild acute knee pain of less than 3 months duration in otherwise healthy adults. The study was an open, dose-ranging postal study in volunteers who had been recruited through newspaper and magazine articles. Two validated questionnaires (WOMAC knee health Index and the Psychological Well-Being Index) were completed at baseline and after one month's intervention with bromelain, randomly allocated to volunteers as either 200 mg or 400 mg per day. Seventy seven subjects completed the study. In both treatment groups, all WOMAC symptom dimension scores were significantly reduced compared with baseline, with reductions in the final battery (total symptom score) of 41 and 59% (P = 0.0001 and <0.0001) in the low and high dose groups respectively. In addition, improvements in total symptom score (P = 0.036) and the stiffness (P = 0.026) and physical function (P = 0.021) dimensions were significantly greater in the high-dose (400 mg per day) compared with the low-dose group. Compared to baseline, overall psychological well-being was significantly improved in both groups after treatment (P = 0.015 and P = 0.0003 in the low and high dose groups respectively), and again, a significant dose-response relationship was observed. We conclude that bromelain may be effective in ameliorating physical symptoms and improving general well-being in otherwise healthy adults suffering from mild knee pain in a dose-dependant manner. Double blind, placebo-controlled studies are now warranted to confirm these results.
9) Ley CM, Tsiami A, Ni Q, Robinson N. A review of the use of bromelain in cardiovascular diseases. Zhong Xi Yi Jie He Xue Bao. 2011 Jul;9(7):702-10.
Source
Social Care and Human Sciences, School of Psychology, University of West London, Middlesex, TW8 9GA, UK.
Abstract
BACKGROUND:
In 2004 an estimated 17.1 million people died from cardiovascular diseases (CVDs) worldwide, representing 29% of all global deaths. According to the American Heart Association, heart disease and stroke are the main cause of death and disability among people with type 2 diabetes. Additional safe and effective approaches are needed for the prevention and management of CVDs which may include nutritional supplements.
OBJECTIVE:
To identify the potential of bromelain (a food supplement) on the risk factors associated with CVDs.
SEARCH STRATEGY:
An electronic and manual search was conducted during November 2009 to March 2010. The databases searched included: Ovid MEDLINE; All EBM Reviews-Cochrane Database of Systematic Reviews (Cochrane DSR), American College of Physicians (ACP) Journal Club, Database of Abstracts of Reviews of Effects (DARE), Cochrane Central Register of Controlled Trials (CCTR), Cochrane Methodology Register (CMR), Health Technology Assessment (HTA) and National Health Service Economic Evaluation Database (NHSEED); Allied and Complementary Medicine (AMED); British Nursing Index and Archive; EMBASE; Health Management Information Consortium (HMIC); ScienceDirect and Electronic Thesis Online Services (ETHOS). Only papers in the English language were included.
INCLUSION CRITERIA:
Randomised controlled trials (RCTs), human studies, animal studies and experimental studies related to bromelain for CVDs. Data extraction and analysis: The quality assessment of all the selected studies was conducted by the authors. Data from 3 animal trials and 3 human trials were included in the review. Data collected included: type of trial, drug dosage, duration, outcome measures, characteristics of bromelain used, significance of results and conclusion.
RESULTS:
Out of 223 papers retrieved, 6 papers met the inclusion criteria and could be included in the review. These comprised of 3 animal and 3 human trials, each of which investigated the use of bromelain for CVDs. Results suggested that bromelain could be used for treating acute thrombophlebitis, as it decreases aggregation of blood platelets, has a cardio-protective effect, ameliorates rejection-induced arterial wall remodelling, prevents thrombin-induced human platelet aggregation as well as reduces thrombus formation.
CONCLUSION:
No substantive study of bromelain and clinical CVDs has been carried out in human populations. Only a few studies on bromelain and CVDs were published from 1948 to 2010. This may be an area worthy to be explored in future CVDs research.
10) Loskutoff DJ, Quigley JP. PAI-1, fibrosis, and the elusive provisional fibrin matrix. J Clin Invest. 2000 Dec;106(12):1441-3.
Whether induced surgically or by hypertension, infections, extreme heat, or caustic chemicals, tissue injury invariably leads to vasodilatation, with subsequent leakage of plasma proteins into the connective tissues, rapid activation of the coagulation cascade, and deposition of fibrin. A central paradigm in the field is that the fibrin is organized into a "provisional fibrin matrix," which acts as a road map to direct the migration of invading cells. Leukocytes and possibly fibroblasts migrate into the area and elaborate cytokines which, in turn, stimulate resident cells to synthesize and deposit collagens and other insoluble fibrillar components into the evolving extracellular matrix (ECM).
Fibrotic disease occurs when normal control of this process is compromised and excess fibrous material accumulates in the tissues. It is generally assumed that the persistence of fibrin in the matrix promotes fibrosis, and that the extent of fibrosis is limited by that remove the fibrin (i.e., the fibrinolytic system). In a recent issue of the JCI, Hattori et al. (1) affirm previous suggestions that plasminogen activator inhibitor-1 (PAI-1) promotes pathological fibrosis but challenges the concept that fibrin is required.
11) Felton GE. Fibrinolytic and antithrombotic action of bromelain may eliminate thrombosis in heart patients. Med Hypotheses. 1980 Nov;6(11):1123-33.
Abstract
It has been established that a bromelain plasminogen activator will produce plasmin in rat experiments. In addition the plasmin cleaves Hageman factor in a way that leads to a strong release of kallikrein but a weak release of thrombin. A possible mechanism is suggested to explain how the body can maintain thrombin at a level too low to cause platelet aggregation but adequate to stimulate release of prostaglandins and enzymes for more than 24 hours from a single dose of the pineapple enzymes. Since bromelain therapy leads to formation of platelets with increased resistance to aggregation, it is obvious that the dominant endogenous prostaglandins being produced must be from the group that increases platelet cyclicAMP levels (prostacyclin, PGE1, etc.). The combination of fibrinolytic and antithrombic properties appear to be effective and two large scale tests on heart patients have shown a practically complete elimination of thrombosis.
12) Bracale G, Selvetella L. [Clinical study of the efficacy of and tolerance to seaprose S in inflammatory venous disease. Controlled study versus serratio-peptidase]. Minerva Cardioangiol. 1996 Oct;44(10):515-24.
[Article in Italian]
Source
Divisione di Chirurgia Vascolare, Università degli Studi di Napoli, Federico II.
Abstract
This study was designed to compare the efficacy and safety of seaprose S and serratio-peptidase in the treatment of venous inflammatory disease. Forty patients entered the study (11 males, 29 females), mean age 54.3 years (range 30-77), mean weight 74.8 kg (range 51-96), with superficial thrombophlebitis. The trial was conducted following a controlled, between patients, randomized experimental design. Seaprose S was administered as 30 mg tablets at a daily dosage of 90 mg (one tab t.i.d.), and serratio-peptidase as 5 mg tablets, at a dose of 30 mg per day (two tabs t.i.d.), both orally, for 14 days. Twenty patients received seaprose S and 20 serratio-peptidase. The findings indicate that seaprose S was more effective and better tolerated than serratio-peptidase. Although the group of patients assigned to seaprose S had considerably more severe initial symptoms, by the end of treatment spontaneous pain was reduced 68.7% from the baseline mean score (from 3.2 to 1.0), as compared with a 63.3% reduction in the serratio-peptidase group (from 3.0 to 1.1). Pain on pressure was reduced 61.1% with seaprose S (from 3.6 to 1.4), compared to 57.6% with the reference treatment (from 3.3 to 1.4). Edema was reduced respectively 75% (from 1.6 to 0.4) and 56.2% (from 1.6 to 0.7); erythema diminished 72.4% (from 2.9 to 0.8) and 58.3% (from 2.4 to 1.0); nighttime cramps were 61.1% less (from 1.8 to 0.7) compared with 52.9% (from 1.7 to 0.8); hemorrhagic suffusion was 53.3% less (from 1.5 to 0.7) compared with 41.7% (from 1.2 to 0.7); cutaneous dystrophy was reduced by 11.1% (from 1.8 to 1.6) and 7.7% (from 1.3 to 1.2). At the end of the treatment with seaprose S efficacy was assessed as good or excellent in 85% of the cases, compared with 65% for serratio-peptidase. Seaprose S caused no adverse reactions. During serratio-peptidase treatment one patient reported diarrhea, requiring temporary dosage reduction and specific treatment. It can thus be confirmed that seaprose S was effective and well tolerated in patients with inflammatory venous diseases.
13) Braga PC, Moretti M, Piacenza A, Montoli CC, Guffanti EE. Effects of seaprose on the rheology of bronchial mucus in patients with chronic bronchitis. A double-blind study vs placebo. Int J Clin Pharmacol Res. 1993;13(3):179-85.
Source
Centre for Respiratory Pharmacology, School of Medicine, University of Milan, Italy.
Abstract
There are changes in the rheological characteristics of mucus (viscoelasticity) in several pulmonary pathologies, and especially in chronic bronchitis. Seaprose, a proteolytic enzyme, is one of the pharmacological possibilities for affecting the rheology of bronchial mucus to correct mucostasis and improve its clearance. The action of this drug on the viscoelasticity of bronchial mucus was assessed in a double-blind vs placebo study with 20 randomly balanced chronic bronchitis patients using a new kind of portable rheometer with special features designed for routine bronchial mucus analysis in clinical practice at the patient's bedside. It was found that in the group of patients who were given the placebo, there were no particular changes in the rheological behaviour of mucus, while in those patients who were given seaprose there were significant changes in both viscosity and elasticity at the end of treatment. Eight days after the end of treatment with seaprose, there was still a significant beneficial effect on the viscoelasticity of mucus and a sort of "post-mucolytic effect" can be postulated. Seaprose also had antiinflammatory action, and since in chronic bronchitis there are variable degrees of inflammations, its beneficial long-lasting effect could also be ascribed to this concomitant action.
14) Moretti M, Bertoli E, Bulgarelli S, Testoni C, Guffanti EE, Marchioni CF, Braga PC. Effects of seaprose on sputum biochemical components in chronic bronchitic patients: a double-blind study vs placebo. Int J Clin Pharmacol Res. 1993;13(5):275-80.
Source
Istituto di Tisiologia e Malattie dell' Apparato Respiratorio, Università di Modena, Italy.
Abstract
Seaprose is a semialkaline proteinase endowed with proteolytic effect and antiinflammatory activity tested in different clinical trials. There is clinical evidence that seaprose reduces sputum viscoelastic properties in chronic hypersecretory bronchitis. The present study evaluated (in a double-blind design vs. placebo) the activity of seaprose on bronchial inflammation, mucus glycoprotein secretion and bronchial humoral defence mechanism in chronic bronchitic patients clinically stable (10 per group). Markers of bronchial inflammation (albumin, albumin/total protein ratio) and bronchial infection (DNA), of mucus glycoproteins (fucose and N-acetylneuraminic acid) and of humoral defence mechanism (secretory-IgA) were tested in sputum. We found that ten-day treatment with seaprose (90 mg/day) reduced sputum albumin during the observation period, the difference being statistically significant at the 18th day. The sputum albumin/total protein ratio also decreased by 50% at the end of the study. In the same group, sputum DNA, secretory-IgA, fucose and N-acetylneuraminic acid remained unchanged after treatment. The placebo group did not show any significant changes in the sputum marker substances. This study provides experimental evidence for the antiinflammatory activity of seaprose on bronchial mucosa in chronic bronchitic patients studied in a stable phase of their disease. Furthermore the drug does not seem to affect mucus glycoprotein secretion or secretory-IgA production.
15) Fitzhugh DJ, Shan S, Dewhirst MW, Hale LP.Bromelain treatment decreases neutrophil migration to sites of inflammation. Clin Immunol. 2008 Jul;128(1):66-74.
Source
Department of Pathology, DUMC 3712, Duke University Medical Center, Durham, NC 27710, USA.
Abstract
Bromelain, a mixture of proteases derived from pineapple stem, has been reported to have therapeutic benefits in a variety of inflammatory diseases, including murine inflammatory bowel disease. The purpose of this work was to understand potential mechanisms for this anti-inflammatory activity. Exposure to bromelain in vitro has been shown to remove a number of cell surface molecules that are vital to leukocyte trafficking, including CD128a/CXCR1 and CD128b/CXCR2 that serve as receptors for the neutrophil chemoattractant IL-8 and its murine homologues. We hypothesized that specific proteolytic removal of CD128 molecules by bromelain would inhibit neutrophil migration to IL-8 and thus decrease acute responses to inflammatory stimuli. Using an in vitro chemotaxis assay, we demonstrated a 40% reduction in migration of bromelain- vs. sham-treated human neutrophils in response to rhIL-8. Migration to the bacterial peptide analog fMLP was unaffected, indicating that bromelain does not induce a global defect in leukocyte migration. In vivo bromelain treatment generated a 50-85% reduction in neutrophil migration in 3 different murine models of leukocyte migration into the inflamed peritoneal cavity. Intravital microscopy demonstrated that although in vivo bromelain treatment transiently decreased leukocyte rolling, its primary long-term effect was abrogation of firm adhesion of leukocytes to blood vessels at the site of inflammation. These changes in adhesion were correlated with rapid re-expression of the bromelain-sensitive CD62L/L-selectin molecules that mediate rolling following in vivo bromelain treatment and minimal re-expression of CD128 over the time period studied. Taken together, these studies demonstrate that bromelain can effectively decrease neutrophil migration to sites of acute inflammation and support the specific removal of the CD128 chemokine receptor as a potential mechanism of action.
16) Berg A, Peters M, Deibert P, König D, Birnesser H. Bromelain - Overview and diskussion of therapeutic application and its importance in sports medicine and sports traumatology. Deutsche Zeitschrift Für Sportmedizin. Jahrgang 56, Nr. 1 (2005) [German]
Bromelain, a plant-derived proteolytic enzyme, is commercially available and has been approved as a pharmaceutical preparation. Bromelain is mainly prescribed for the treatment and prevention of inflammatory, posttraumatic or postoperative swelling. The mode of action has been investigated in cell-culture and animal experiments. In controlled clinical studies in humans, orally-administered Bromelain has proven its
pharmaceutical efficacy by significantly reducing soft-tissue edema in the above-mentioned conditions. Therefore, Bromelain is also of interest for sports medicine and sports traumatology. Oral treatment with Bromelain has few and only transient and mild side-effects and may therefore be an effective alternative for non-steroidal anti-inflammatory drugs in the treatment of posttraumatic edema and swelling. This review summarizes present knowledge regarding the mode of action of Bromelain and gives an overview about its practical applications from a sports-medical point of view.
17) Hong J, Bose M, Ju J, Ryu JH, Chen X, Sang S, Lee MJ, Yang CS. Modulation of arachidonic acid metabolism by curcumin and related beta-diketone derivatives: effects on cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxygenase. Carcinogenesis. 2004 Sep;25(9):1671-9
Source
Susan Lehman Cullman Laboratory for Cancer Research, Department of Chemical Biology, Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, Piscataway, NJ 08854, USA.
Abstract
Aberrant arachidonic acid metabolism is involved in the inflammatory and carcinogenic processes. In this study, we investigated the effects of curcumin, a naturally occurring chemopreventive agent, and related beta-diketone derivatives on the release of arachidonic acid and its metabolites in the murine macrophage RAW264.7 cells and in HT-29 human colon cancer cells. We also examined their effects on the catalytic activities and protein levels of related enzymes: cytosolic phospholipase A(2) (cPLA(2)), cyclooxygenases (COX) as well as 5-lipoxygenase (5-LOX). At 10 micro M, dibenzoylmethane (DBM), trimethoxydibenzoylmethane (TDM), tetrahydrocurcumin (THC) and curcumin effectively inhibited the release of arachidonic acid and its metabolites in lipopolysaccharide (LPS)-stimulated RAW cells and A23187-stimulated HT-29 cells. Inhibition of phosphorylation of cPLA(2), the activation process of this enzyme, rather than direct inhibition of cPLA(2) activity appears to be involved in the effect of curcumin. All the curcuminoids (10 micro M) potently inhibited the formation of prostaglandin E(2) (PGE(2)) in LPS-stimulated RAW cells. Curcumin (20 micro M) significantly inhibited LPS-induced COX-2 expression; this effect, rather than the catalytic inhibition of COX, may contribute to the decreased PGE(2) formation. Without LPS-stimulation, however, curcumin increased the COX-2 level in the macrophage cells. Studies with isolated ovine COX-1 and COX-2 enzymes showed that the curcuminoids had significantly higher inhibitory effects on the peroxidase activity of COX-1 than that of COX-2. Curcumin and THC potently inhibited the activity of human recombinant 5-LOX, showing estimated IC(50) values of 0.7 and 3 micro M, respectively. The results suggest that curcumin affects arachidonic acid metabolism by blocking the phosphorylation of cPLA(2), decreasing the expression of COX-2 and inhibiting the catalytic activities of 5-LOX. These activities may contribute to the anti-inflammatory and anticarcinogenic actions of curcumin and its analogs.
18) Sreejayan, Rao MN. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol. 1997 Jan;49(1):105-7.
Source
Department of Pharmaceutical Chemistry, College of Pharmaceutical Sciences, Manipal, India.
Abstract
Because curcumin, a compound with anti-inflammatory and anticancer activity, inhibits induction of nitric oxide synthase in activated macrophages and has been shown to be a potent scavenger of free radicals we have investigated whether it can scavenge nitric oxide directly. Curcumin reduced the amount of nitrite formed by the reaction between oxygen and nitric oxide generated from sodium nitroprusside. Other related compounds, e.g. demethoxycurcumin, bisdemethoxycurcumin and diacetylcurcumin were as active as curcumin, indicating that the methoxy and the phenolic groups are not essential for the scavenging activity. The results indicate curcumin to be a scavenger of nitric oxide. Because this compound is implicated in inflammation and cancer, the therapeutic properties of curcumin against these conditions might be at least partly explained by its free-radical scavenging properties, including those toward nitric oxide.
19) Otsuki N, Dang NH, Kumagai E, Kondo A, Iwata S, Morimoto C. Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. J Ethnopharmacol. 2010 Feb 17;127(3):760-7.
Source
Division of Clinical Immunology, Advanced Clinical Research Center, The Institute of Medical Science, The University of Tokyo, Tokyo, Japan.
Abstract
AIM OF THE STUDY:
Various parts of Carica papaya Linn. (CP) have been traditionally used as ethnomedicine for a number of disorders, including cancer. There have been anecdotes of patients with advanced cancers achieving remission following consumption of tea extract made from CP leaves. However, the precise cellular mechanism of action of CP tea extracts remains unclear. The aim of the present study is to examine the effect of aqueous-extracted CP leaf fraction on the growth of various tumor cell lines and on the anti-tumor effect of human lymphocytes. In addition, we attempted to identify the functional molecular weight fraction in the CP leaf extract.
MATERIALS AND METHODS:
The effect of CP extract on the proliferative responses of tumor cell lines and human peripheral blood mononuclear cells (PBMC), and cytotoxic activities of PBMC were assessed by [(3)H]-thymidine incorporation. Flow cytometric analysis and measurement of caspase-3/7 activities were performed to confirm the induction of apoptosis on tumor cells. Cytokine productions by PBMC were measured by ELISA. Gene profiling of the effect of CP extract treatment was performed by microarray analysis and real-time RT-PCR.
RESULTS:
We observed significant growth inhibitory activity of the CP extract on tumor cell lines. In PBMC, the production of IL-2 and IL-4 was reduced following the addition of CP extract, whereas that of IL-12p40, IL-12p70, IFN-gamma and TNF-alpha was enhanced without growth inhibition. In addition, cytotoxicity of activated PBMC against K562 was enhanced by the addition of CP extract. Moreover, microarray analyses showed that the expression of 23 immunomodulatory genes, classified by gene ontology analysis, was enhanced by the addition of CP extract. In this regard, CCL2, CCL7, CCL8 and SERPINB2 were representative of these upregulated genes, and thus may serve as index markers of the immunomodulatory effects of CP extract. Finally, we identified the active components of CP extract, which inhibits tumor cell growth and stimulates anti-tumor effects, to be the fraction with M.W. less than 1000.
CONCLUSION:
Since Carica papaya leaf extract can mediate a Th1 type shift in human immune system, our results suggest that the CP leaf extract may potentially provide the means for the treatment and prevention of selected human diseases such as cancer, various allergic disorders, and may also serve as immunoadjuvant for vaccine therapy.
20) Gayathri B, Manjula N, Vinaykumar KS, Lakshmi BS, Balakrishnan A. Pure compound from Boswellia serrata extract exhibits anti-inflammatory property in human PBMCs and mouse macrophages through inhibition of TNFalpha, IL-1beta, NO and MAP kinases. Int Immunopharmacol. 2007 Apr;7(4):473-82. Source
Centre for Biotechnology, Anna University, Chennai, India.
Abstract
The aim of the present study is to probe the anti-inflammatory potential of the plant Boswellia serrata by studying the effect of the crude extract and the pure compound isolated from it on key inflammatory mediators like TNFalpha, IL-1beta, and NO thus enabling the understanding of the key signaling events involved. The crude methanolic extract and the pure compound were analysed for their inhibitory effect on TNFalpha, IL-1beta and IL-6. The results demonstrated that all three cytokines are down regulated when PBMCs are cultured in the presence of crude extract or the pure compound at various time points. Observations on Th1/Th2 cytokines revealed marked down regulation of Th1 cytokines IFNgamma and IL-12 while the Th2 cytokines IL-4 and IL-10 were up regulated upon treatment with crude extract and pure compound. The extract and the pure compound isolated also showed considerable inhibition of NO production in activated RAW 264.7 cells, possibly via suppression of inducible NO synthase mRNA expression. Further to elucidate the underlying mechanism of action the effect of 12-ursene 2-diketone on LPS-induced activation of MAPK has also been examined. Our results demonstrated that 12-ursene 2-diketone inhibits the expression of pro-inflammatory cytokines and mediators via inhibition of phosphorylation of the MAP kinases JNK and p38 while no inhibition was seen in ERK phosphorylation in LPS-stimulated PBMCs. The above study therefore indicates that the crude methanolic extract and the isolated pure compound are capable of carrying out a natural anti-inflammatory activity at sites where chronic inflammation is present by switching off the pro-inflammatory cytokines and mediators, which initiate the process.
21) Kokkiripati PK, Bhakshu LM, Marri S, Padmasree K, Row AT, Raghavendra AS, Tetali SD. Gum resin of Boswellia serrata inhibited human monocytic (THP-1) cell activation and platelet aggregation. J Ethnopharmacol. 2011 Sep 1;137(1):893-901.
Source
Department of Plant Sciences, University of Hyderabad, Hyderabad 500046, India.
Abstract
ETHNOPHARMACOLOGICAL RELEVANCE:
Stem bark gum resin extract of Boswellia serrata is traditionally used in India for its hemostatic, antiinflammatory and cardiovascular health effects and it is named as Śallakī in Ayurvedic medicine.
AIM OF THE STUDY:
This study was conducted to evaluate the antioxidative and antithrombotic properties of stem bark gum resin extracts of Boswellia serrata (BS).
MATERIALS AND METHODS:
The inhibitory activity of the BSWE and BSAE on FeCl(3) induced lipid peroxidation (in vitro) in rat liver and heart homogenates was measured spectrophotometrically. Their effect on H(2)O(2) induced reactive oxygen species (ROS) generation in human monocytic (THP-1) cells was investigated by tracking intensity of a cell permeable fluorescent dye, H(2)DCFDA and subjecting the cell samples to confocal microscopy. Further, the effect of BSAE and BSWE on ADP-induced platelet aggregation was assessed using a multimode detection plate reader, plasma coagulation times using an automated blood coagulation analyzer and on human blood clotting factors Xa and XIa using chromogenic substrate. Phytomarker analysis of the water (BSWE) and hydroalcoholic (BSAE) extracts of BS-gum resin was done through HPLC using a standard compound AKβBA.
RESULTS:
BSAE and BSWE inhibited, to varied extents, the lipid peroxidation in liver (80%) and heart (50%) tissue homogenates of male Wistar rats. Further, BSAE (30 μg dwt/mL) and BSWE (300 μg dwt/mL) attenuated ≥ 60% of H(2)O(2) mediated ROS generation in THP-1 cells. In case of standard compounds, ascorbate (20 μg dwt/mL) and butylated hydroxytoluene (BHT) (10 μg dwt/mL) completely scavenged ROS in the cells. BSAE and BSWE at 3 mg dwt/mL completely inhibited ADP induced platelet aggregation and activities were comparable to 20 μg/mL of heparin. The extracts also showed very high activity in prolonging coagulation time periods. Both types of extracts extended prothrombin time (PT) from ∼13 to >60s and activated partial thromboplastin time (APTT) from ∼32s to >90s. BSAE inhibited clotting factors Xa and XIa remarkably at 6 μg of dwt where as BSWE did not show much effect on FXa and showed 30% inhibition on FXIa at 120 μg. 10 μg of heparin was required to inhibit about 30% activity of the above factors. HPLC analyses suggested that BSAE and BSWE had AKβBA of 9% (w/w) and 7.8% (w/w) respectively.
CONCLUSION:
Present study demonstrated antioxidant and antithrombotic anticoagulant activities of water and hydroalcoholic extracts of Boswellia serrata's gum resin. We suggest that BS-gum resin as a good source for lead/therapeutic compounds possessing antioxidant, antiplatelet and anticoagulant activities.
22) Piacente S, Montoro P, Oleszek W, Pizza C. Yucca schidigera bark: phenolic constituents and antioxidant activity. J Nat Prod. 2004 May;67(5):882-5.
Source
Dipartimento di Scienze Farmaceutiche, Università degli Studi di Salerno, Via Ponte Don Melillo, 84084 Fisciano, Salerno, Italy.
Abstract
Two new phenolic constituents with unusual spirostructures, named yuccaols D (1) and E (2), were isolated from the MeOH extract of Yucca schidigera bark. Their structures were established by spectroscopic (ESIMS and NMR) analysis. The new yuccaols D and E, along with resveratrol (3), trans-3,3',5,5'-tetrahydroxy-4'-methoxystilbene (4), yuccaols A-C (5-7), yuccaone A (8), larixinol (9), the MeOH extract of Yucca schidigera bark, and the phenolic portion of this extract, were assayed for antioxidant activity by measuring the free radical scavenging effects using two different assays, namely, the Trolox Equivalent Antioxidant Capacity (TEAC) assay and the coupled oxidation of beta-carotene and linoleic acid (autoxidation assay). The significant activities exhibited by the phenolic fraction and its constituents in both tests show the potential use of Y. schidigera as a source of antioxidant principles.
23) Cheeke PR, Piacente S, Oleszek W. Anti-inflammatory and anti-arthritic effects of Yucca schidigera: a review. J Inflamm (Lond). 2006 Mar 29;3:6.
Source
Department of Animal Sciences, Oregon State University, Corvallis, OR 97333, USA. peter.r.cheeke@oregonstate.edu
Abstract
Yucca schidigera is a medicinal plant native to Mexico. According to folk medicine, yucca extracts have anti-arthritic and anti-inflammatory effects. The plant contains several physiologically active phytochemicals. It is a rich source of steroidal saponins, and is used commercially as a saponin source. Saponins have diverse biological effects, including anti-protozoal activity. It has been postulated that saponins may have anti-arthritic properties by suppressing intestinal protozoa which may have a role in joint inflammation. Yucca is also a rich source of polyphenolics, including resveratrol and a number of other stilbenes (yuccaols A, B, C, D and E). These phenolics have anti-inflammatory activity. They are inhibitors of the nuclear transcription factor NFkappaB. NFkB stimulates synthesis of inducible nitric oxide synthase (iNOS), which causes formation of the inflammatory agent nitric oxide. Yucca phenolics are also antioxidants and free-radical scavengers, which may aid in suppressing reactive oxygen species that stimulate inflammatory responses. Based on these findings, further studies on the anti-arthritic effects of Yucca schidigera are warranted.
THE PHENOLS FOUND IN YUCCA SCHIDIGERA HAVE ANTI-INFLAMMATORY ACTIVITIES AND INHIBIT NITRIC OXIDE, WHICH IS AN INFLAMMATORY AGENT. THEY ALSO HAVE ANTIOXIDANT ACTIVITIES WHICH MAY MODULATE INFLAMMATORY RESPONSE. FULL STUDY AVAILABLE HERE:
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1440857/ Anti-inflammatory and anti-arthritic effects of yucca schidigera: A review (FULL STUDY)
24) Grzanna R, Lindmark L, Frondoza CG. Ginger--an herbal medicinal product with broad anti-inflammatory actions. J Med Food. 2005 Summer;8(2):125-32.
Source
RMG Biosciences, Inc.
Abstract
The anti-inflammatory properties of ginger have been known and valued for centuries. During the past 25 years, many laboratories have provided scientific support for the long-held belief that ginger contains constituents with antiinflammatory properties. The original discovery of ginger's inhibitory effects on prostaglandin biosynthesis in the early 1970s has been repeatedly confirmed. This discovery identified ginger as an herbal medicinal product that shares pharmacological properties with non-steroidal anti-inflammatory drugs. Ginger suppresses prostaglandin synthesis through inhibition of cyclooxygenase-1 and cyclooxygenase-2. An important extension of this early work was the observation that ginger also suppresses leukotriene biosynthesis by inhibiting 5-lipoxygenase. This pharmacological property distinguishes ginger from nonsteroidal anti-inflammatory drugs. This discovery preceded the observation that dual inhibitors of cyclooxygenase and 5-lipoxygenase may have a better therapeutic profile and have fewer side effects than non-steroidal anti-inflammatory drugs. The characterization of the pharmacological properties of ginger entered a new phase with the discovery that a ginger extract (EV.EXT.77) derived from Zingiber officinale (family Zingiberaceae) and Alpina galanga (family Zingiberaceae) inhibits the induction of several genes involved in the inflammatory response. These include genes encoding cytokines, chemokines, and the inducible enzyme cyclooxygenase-2. This discovery provided the first evidence that ginger modulates biochemical pathways activated in chronic inflammation. Identification of the molecular targets of individual ginger constituents provides an opportunity to optimize and standardize ginger products with respect to their effects on specific biomarkers of inflammation. Such preparations will be useful for studies in experimental animals and humans.
25) Ernst E, Pittler MH. Efficacy of ginger for nausea and vomiting: a systematic review of randomized clinical trials. Br J Anaesth. 2000 Mar;84(3):367-71.
Source
Department of Complementary Medicine, School of Postgraduate Medicine and Health Sciences, University of Exeter, UK.
Abstract
Ginger (Zingiber officinale) is often advocated as beneficial for nausea and vomiting. Whether the herb is truly efficacious for this condition is, however, still a matter of debate. We have performed a systematic review of the evidence from randomized controlled trials for or against the efficacy of ginger for nausea and vomiting. Six studies met all inclusion criteria and were reviewed. Three on postoperative nausea and vomiting were identified and two of these suggested that ginger was superior to placebo and equally effective as metoclopramide. The pooled absolute risk reduction for the incidence of postoperative nausea, however, indicated a non-significant difference between the ginger and placebo groups for ginger 1 g taken before operation (absolute risk reduction 0.052 (95% confidence interval -0.082 to 0.186)). One study was found for each of the following conditions: seasickness, morning sickness and chemotherapy-induced nausea. These studies collectively favoured ginger over placebo.
26) Huang TH, Tran VH, Duke RK, Tan S, Chrubasik S, Roufogalis BD, Duke CC. Harpagoside suppresses lipopolysaccharide-induced iNOS and COX-2 expression through inhibition of NF-kappa B activation. J Ethnopharmacol. 2006 Mar 8;104(1-2):149-55.
Source
Pharmaceutical Chemistry and Herbal Medicines Research and Education Centre, Faculty of Pharmacy A15, University of Sydney, NSW 2006, Australia.
Abstract
Preparations of Harpagophytum procumbens, known as devil's claw, are used as an adjunctive therapy for the treatment of pain and osteoarthritis. Pharmacological evaluations have proven the effectiveness of this herbal drug as an anti-inflammatory and analgesic agent. The present study has investigated the mechanism of action of harpagoside, one of the major components of Harpagophytum procumbens, using human HepG2 hepatocarcinoma and RAW 264.7 macrophage cell lines. Harpagoside inhibited lipopolysaccharide-induced mRNA levels and protein expression of cyclooxygenase-2 and inducible nitric oxide in HepG2 cells. These inhibitions appeared to correlate with the suppression of NF-kappaB activation by harpagoside, as pre-treating cells with harpagoside blocked the translocation of NF-kappaB into the nuclear compartments and degradation of the inhibitory subunit IkappaB-alpha. Furthermore, harpagoside dose-dependently inhibited LPS-stimulated NF-kappaB promoter activity in a gene reporter assay in RAW 264.7 cells, indicating that harpagoside interfered with the activation of gene transcription. These results suggest that the inhibition of the expression of cyclooxygenase-2 and inducible nitric oxide by harpagoside involves suppression of NF-kappaB activation, thereby inhibiting downstream inflammation and subsequent pain event.
27) Kidd P. Glutathione: Systemic Protectant Against Oxidative and Free Radical Damage. Alt Med Rev. 1997; Vol.2 (3):155-76.
ABSTRACT
The tripeptide thiol glutathione (GSH) has facile electron-donating capacity, linked to its sulfhydryl (―SH) group. Glutathione is an important water-phase antioxidant and essential cofactor for antioxidant enzymes; it provides protection also for the mitochondria against endogenous oxygen radicals. Its high electron-donating capacity combined with its high intracellular concentration endows GSH with great reducing power, which is used to regulate a complex thiol-exchange system (―SH ↔ ―S-S―). This functions at all levels of cell activity, from the relatively simple (circulating cysteine/―SH thiols, ascorbate, other small molecules) to the most complex (cellular ―SH proteins).
Glutathione is homeostatically controlled, both inside the cell and outside. Enzyme systems synthesize it, utilize it, and regenerate it as per the gamma-glutamyl cycle. Glutathione is most concentrated in the liver (10 mM), where the "P450 Phase II" enzymes require it to convert fat-soluble substances into water-soluble GSH conjugates, in order to facilitate their excretion. While providing GSH for their specific needs, the liver parenchymal cells export GSH to the outside, where it serves as systemic source of ―SH/reducing power.
GSH depletion leads to cell death, and has been documented in many degenerative conditions. Mitochondrial GSH depletion may be the ultimate factor determining vulnerability to oxidant attack. Oral ascorbate helps conserve GSH; cysteine is not a safe oral supplement, and of all the oral GSH precursors probably the least flawed and most cost-effective NAC (N-acetylcysteine). FULL STUDY AVAILABLE HERE: http://www.dockidd.com/pdf/KiddGSHAMR.pdf
28) Dieber-Rotheneder M, Puhl H, Waeg G, Striegl G, Esterbauer H. Effect of oral supplementation with D-alpha-tocopherol on the vitamin E content of human low density lipoproteins and resistance to oxidation. J Lipid Res. 1991 Aug;32(8):1325-32.
Source
Institute of Biochemistry, University of Graz, Austria.
Abstract
Twelve clinically healthy subjects participated in a vitamin E supplementation study. Eight were given daily dosages of 150, 225, 800, or 1200 IU RRR-alpha-tocopherol for 21 days (two persons per dose) and four received placebo. Prior, during, and after the supplementation period, alpha-tocopherol, gamma-tocopherol, and carotenoids were determined in plasma and low density lipoprotein (LDL). The maximum levels of alpha-tocopherol were 1.7- to 2.5-times the baseline values in plasma and 1.7- to 3.1-times in LDL. A high correlation existed between alpha-tocopherol in plasma and LDL. gamma-Tocopherol significantly decreased in plasma and LDL during vitamin E supplementation. No significant influence on the lipoprotein and lipid status and carotenoid levels of the participants occurred throughout the supplementation. The resistance of LDL against copper-mediated oxidation was also measured. The oxidation resistance of LDL was significantly higher during vitamin E supplementation. However, the efficacy of vitamin E in protecting LDL varied from person to person. The statistical evaluation of all data gave a correlation of r2 = 0.51 between alpha-tocopherol in LDL and the oxidation resistance as measured by the length of the lag-phase preceding the oxidation of LDL. No association was seen between levels of carotenoids and vitamin E in plasma and LDL. The present study clearly shows that in humans the oxidation resistance of LDL can be increased by vitamin E supplementation. FULL STUDY AVAILABLE HERE: http://www.jlr.org/content/32/8/1325.full.pdf
SUPPLEMENTING WITH VITAMIN E REDUCES LDL OXIDATION.
NOTE: 1 IU of tocopherol is defined as ⅔ milligrams of RRR-alpha-tocopherol (formerly named d-alpha-tocopherol)
29) Reaven PD, Khouw A, Beltz WF, Parthasarathy S, Witztum JL. Effect of dietary antioxidant combinations in humans. Protection of LDL by vitamin E but not by beta-carotene. Arterioscler Thromb. 1993 Apr;13(4):590-600.
Source
Department of Medicine, University of California, San Diego, La Jolla 92093-0682.
Abstract
Experimental and epidemiological evidence supports the hypothesis that oxidation of low density lipoprotein (LDL) appears to be important in mediating the atherogenicity of LDL. To test this hypothesis in humans, it will be necessary to perform intervention studies in large populations. We performed two studies to assess the effectiveness of supplementation with beta-carotene and vitamin E, used alone and in combination with each other, and with vitamin C, to protect LDL from oxidation. In phase 1, after a placebo period, eight subjects were given beta-carotene (60 mg/day) for 3 months, then beta-carotene plus vitamin E (1,600 mg/day) for another 3 months, and then beta-carotene plus vitamin E plus vitamin C (2 g/day) for 3 months. During phase 2, beta-carotene and vitamin C were discontinued, and subjects took only vitamin E for 5 months. During each period, LDL samples were isolated, and measurements of susceptibility to oxidation were performed. beta-Carotene levels in LDL increased nearly 20-fold, but LDL susceptibility to oxidation did not change. Addition of vitamin E increased LDL vitamin E levels nearly 2.5-fold, and this decreased LDL oxidation 30-40%. During the vitamin C supplementation period, plasma levels of beta-carotene and vitamin E rose, but only beta-carotene increased in LDL. However, the susceptibility of LDL to oxidation in this period was not decreased further. During phase 2, when subjects took only vitamin E, LDL susceptibility to oxidation was decreased by 50% as measured by thiobarbituric acid-reactive substances, conjugated dienes, and lipid peroxide formation as well as by macrophage degradation. Thus, long-term supplementation with large doses of vitamin E alone, but not beta-carotene, conferred increased protection to LDL in in vitro assays of oxidation. These data should be useful in planning therapeutic strategies to test the antioxidant hypothesis in humans.
30) Boshtam M, Rafiei M, Sadeghi K, Sarraf-Zadegan N. Vitamin E can reduce blood pressure in mild hypertensives. Int J Vitam Nutr Res. 2002 Oct;72(5):309-14.
Source
Isfahan Cardiovascular Research Center, PO Box 81465-1148, Isfahan, Iran.
Abstract
This triple-blind placebo-controlled clinical trial was performed to determine the effects of the anti-oxidant vitamin E on blood pressure and heart rate in patients with mild hypertension. A total of 70 new mild hypertensive subjects (systolic blood pressure, SBP: 140-160 mmHg; diastolic blood pressure, DBP: 90-100 mmHg) without secondary hypertension were selected from among people referred to the Hypertension Unit of Isfahan Cardiovascular Research Center and divided randomly into two groups of drug (DG) and placebo (PG). All subjects were aged from 20 to 60 years old, without any other cardiovascular risk factors. The drug group received vitamin E tablets (200 IU/day) and the placebo group received placebo only for 27 weeks. At the beginning and the end of the study, the blood vitamin E level was measured fluorimetrically in all subjects according to the Hansen and Warwick method [14, 15]. Blood pressure and heart rate were measured at the beginning, during, and at the end of the study. Blood pressure was measured by a physician using one random zero mercury sphygmomanometer. Personal information and dietary habits of subjects were collected by separate questionnaire. At the end of the study, it was found that the vitamin E supplement had caused a remarkable decrease in SBP (-24% in DG versus -1.6% in PG) and a less remarkable decrease in DBP (-12.5% in DG versus -6.2% in PG) (p < 0.05). The change in heart rate was -4.3% in DG, and -14.0% in PG (p < 0.05). It is concluded that a vitamin E supplement of 200 IU/day can be effective in mild hypertensive patients in the long term, probably due to nitric oxide, and improve their blood pressure status. Therefore, vitamin E supplement could be recommended to such patients.
31) Office of Dietary Supplements - National Institutes of Health http://ods.od.nih.gov/factsheets/vitamine/#en6 last accessed 03/12/2012
Vitamin E is a fat-soluble antioxidant that stops the production of ROS formed when fat undergoes oxidation. Scientists are investigating whether, by limiting free-radical production and possibly through other mechanisms, vitamin E might help prevent or delay the chronic diseases associated with free radicals.
In addition to its activities as an antioxidant, vitamin E is involved in immune function and, as shown primarily by in vitro studies of cells, cell signaling, regulation of gene expression, and other metabolic processes [1]. Alpha-tocopherol inhibits the activity of protein kinase C, an enzyme involved in cell proliferation and differentiation in smooth muscle cells, platelets, and monocytes [6]. Vitamin-E–replete endothelial cells lining the interior surface of blood vessels are better able to resist blood-cell components adhering to this surface. Vitamin E also increases the expression of two enzymes that suppress arachidonic acid metabolism, thereby increasing the release of prostacyclin from the endothelium, which, in turn, dilates blood vessels and inhibits platelet aggregation [6].
32) Martha Clare Morris, ScD; Denis A. Evans, MD; Julia L. Bienias, ScD; Christine C. Tangney, PhD; Robert S. Wilson, PhD. Vitamin E and Cognitive Decline in Older Persons. Arch Neurol. 2002;59:1125-1132.
Background Previous studies raise the possibility that antioxidants protect against neurodegenerative diseases.
Objective To examine whether intake of antioxidant nutrients, including vitamin E, vitamin C, and carotene, is associated with reduced cognitive decline with age.
Design Longitudinal population-based study conducted from September 17, 1993, to November 20, 2000, with an average follow-up of 3.2 years.
Patients The patients were 2889 community residents, aged 65 to 102 years, who completed a food frequency questionnaire, on average 18 months after baseline.
Main Outcome Measure Cognitive change as measured by 4 tests (the East Boston Memory Test, which tests immediate and delayed recall; the Mini-Mental State Examination; and the Symbol Digit Modalities Test) at baseline and 3 years for all participants, and at 6 months for 288 randomly selected participants.
Results We used random-effects models to estimate nutrient effects on individual change in the average score of the 4 cognitive tests. The cognitive score declined on average by 5.0 x 10-2 standardized units per year. There was a 36% reduction in the rate of decline among persons in the highest quintile of total vitamin E intake (-4.3 x 10-2 standardized units per year) compared with those in the lowest quintile (-6.7 x 10-2 standardized units per year) (P = .05), in a model adjusted for age, race, sex, educational level, current smoking, alcohol consumption, total calorie (energy) intake, and total intakes of vitamin C, carotene, and vitamin A. We also observed a reduced decline with higher vitamin E intake from foods (P = .03 for trend). There was little evidence of association with vitamin C or carotene intake.
Conclusion Vitamin E intake, from foods or supplements, is associated with less cognitive decline with age.
33) Teixeira S. Bioflavonoids: proanthocyanidins and quercetin and their potential roles in treating musculoskeletal conditions. J Orthop Sports Phys Ther. 2002 Jul;32(7):357-63.
Source
Huber Associates, Auburn, ME 04210, USA. shan.tex@verizon.net
Abstract
As a clinician treating musculoskeletal conditions, one is continually in search of safe and more effective treatment methods that will hasten tissue healing. Chronic inflammation has been shown to cause connective tissue degradation. Typically, nonsteroidal anti-inflammatory drugs (NSAIDs) and/or corticosteroids are used to control the inflammatory process, however, long-term use has been associated with potentially serious side effects. The purpose of this article is to introduce and describe literature on 2 natural compounds, namely, proanthocyanidin (PCO) and quercetin, which are 2 specific types of bioflavonoids, and to discuss their potential benefits in treating musculoskeletal conditions. There is evidence to suggest that flavonoids may be beneficial to connective tissue for several reasons, which include the limiting of inflammation and associated tissue degradation, the improvement of local circulation, as well as the promoting of a strong collagen matrix. An overview of bioflavonoids as well as relevant research, safety issues, absorption, and specific sources of PCO and quercetin in foods and through supplementation is included.
Inflammation and Tissue Degradation
Inflammation is a normal biological process and the primary means by which tissue healing is initiated and infection is limited in the human body. However, chronic inflammation has been shown to cause connective tissue degradation. This occurs through 2 primary mechanisms: (1) proteolytic enzymes and (2) oxygen-free radicals such as superoxide ion (O2), singlet, and hydroxyl radicals.
Proteolytic enzymes including elastase, collagenase, and hyaluronidase are involved in the inflammatory process and are associated with the presence of polymorphonuclear leukocytes (PMNs) and macrophages. They have been shown to be more prevalent in
tissue with chronic conditions and to cause degradation of collagen, elastin, and hyaluronic acid.FULL STUDY AVAILABLE HERE: http://www.osptla.com/JOSPT%20Flavonoid%20Article.pdf
34) Ishita Chattopadhyay, Kaushik Biswas, Uday Bandyopadhyay and Ranajit K. Banerjee. Turmeric and curcumin: Biological actions and medicinal applications.
Current Science, 2004, Jul 10, vol. 87, no. 1
Turmeric (Curcuma longa) is extensively used as a spice, food preservative and colouring material in India, China and South East Asia. It has been used in traditional medicine as a household remedy for various diseases, including biliary disorders, anorexia, cough, diabetic wounds, hepatic disorders, rheumatism and sinusitis. For the last few decades, extensive work has been done to establish the biological activities and pharmacological actions of turmeric and its extracts. Curcumin (diferuloylmethane), the main yellow bioactive component of turmeric has been shown to have a wide spectrum of biological actions. These include its antiinflammatory, antioxidant, anticarcinogenic, antimutagenic, anticoagulant, antifertility, antidiabetic, antibacterial, antifungal, antiprotozoal, antiviral, antifibrotic, antivenom, antiulcer, hypotensive and hypocholesteremic activities. Its anticancer effect is mainly mediated through induction of apoptosis. Its antiinflammatory, anticancer and antioxidant roles may be clinically exploited to control rheumatism, carcinogenesis and oxidative stress-related pathogenesis. Clinically, curcumin has already been used to reduce post-operative inflammation. Safety evaluation studies indicate that both turmeric and curcumin are well tolerated at a very high dose without any toxic effects. Thus, both turmeric and curcumin have the potential for the development of modern medicine for the treatment of various diseases. FULL STUDY AVAILABLE HERE: http://repository.ias.ac.in/5196/1/306.pdf
OTHER RELATED RESEARCH
Hale LP, Greer PK, Sempowski GD. Bromelain treatment alters leukocyte expression of cell surface molecules involved in cellular adhesion and activation. Clin Immunol. 2002 Aug;104(2):183-90.
Source
Department of Pathology, Duke University, Durham, NC 27710, USA.
Abstract
Bromelain is a natural proteinase preparation derived from pineapple stem that is marketed for oral use as a digestive aid and as an antiinflammatory agent. Bromelain treatment in vitro has been previously shown to selectively remove certain cell surface molecules that may affect lymphocyte migration and activation. This study reports the effects of bromelain on a broad range of cell surface molecules and on lymphocytes, monocytes, and granulocytes under physiologically relevant conditions. In vitro bromelain treatment of leukocytes in whole blood proteolytically altered 14 of 59 leukocyte markers studied. Constitutively expressed bromelain-sensitive molecules included CD7, CD8alpha, CD14, CD16, CD21, CD41, CD42a, CD44, CD45RA, CD48, CD57, CD62L, CD128a, and CD128b. The proteolytic effect of bromelain increased as the concentration of plasma decreased, with EC50 ranging from >1000 microg/ml for 100% plasma to approximately 1 microg/ml in the absence of plasma, indicating the presence of an inhibitor of bromelain in plasma. alpha2-macroglobulin purified from plasma mimicked the inhibitory effect of whole plasma on bromelain activity. If proteolysis is required for the antiinflammatory actions of oral bromelain, these data suggest that the required concentrations are more likely to be achieved locally in the gastrointestinal tract or in other tissue sites where the plasma concentration is low, rather than in the bloodstream. The cell surface molecules altered by bromelain are involved in leukocyte homing and cellular adhesion and activation. Thus bromelain could potentially exert an antiinflammatory effect by multiple mechanisms, including alterations in leukocyte migration and activation.
THE ANTIINFLAMMATORY EFFECT OF BROMELAIN MAY BE CAUSED BY AN ALTERATION IN LEUKOCYTE MIGRATION AND ACTIVATION.
Masson M. [Bromelain in blunt injuries of the locomotor system. A study of observed applications in general practice]. Fortschr Med. 1995 Jul 10;113(19):303-6.
[Article in German]
Abstract
METHOD: In an open case observation study involving patients with blunt injuries to the musculoskeletal system, the efficacy and tolerability of high-dose Bromelain POS, a plant-derived enzyme preparation, were investigated. The investigating physician was an orthopedic surgeon who, in addition to the usual therapeutic measures, treated 59 of his patients with the bromelaine preparation. The duration of the application was determined by the nature and severity of the lesion, and varied between one and three weeks. The test criteria were swelling, pain at rest and during movement, and tenderness. These parameters were evaluated on the day of the injury and on five subsequent dates. RESULTS: Treatment with bromelaine resulted in a clear reduction in all four parameters tested. Both swelling and the symptoms of pain had improved appreciably at all evaluation time points as compared with baseline. The tolerability of the preparation was very good, and patient compliance was correspondingly high.
What was dosage? Where is full study? What is bromelain pos?
Brien S, Lewith G, Walker A, Hicks SM, Middleton D. Bromelain as a Treatment for Osteoarthritis: a Review of Clinical Studies. Evid Based Complement Alternat Med. 2004 Dec;1(3):251-257.
Abstract
Bromelain, an extract from the pineapple plant, has been demonstrated to show anti-inflammatory and analgesic properties and may provide a safer alternative or adjunctive treatment for osteoarthritis. All previous trials, which have been uncontrolled or comparative studies, indicate its potential use for the treatment of osteoarthritis. This paper reviews the mechanism of its putative therapeutic actions, those clinical trials that have assessed its use in osteoarthritis to date, as well as considering the safety implications of this supplement for osteoarthritis and reviewing the evidence to date regarding the dosage for treating this condition. The data available at present indicate the need for trials to establish the efficacy and optimum dosage for bromelain and the need for adequate prospective adverse event monitoring in such chronic conditions as osteoarthritis.
Find full study. Not enough here to draw conclusions.
Fossati A. Antiinflammatory effects of seaprose-S on various inflammation models. Drugs Exp Clin Res. 1999;25(6):263-70.
Abstract
The antiinflammatory activity of seaprose-S in different experimental models involving different biochemical mediators of inflammation was investigated. In vivo experiments were performed using male Sprague-Dawley rats and in vitro experiments were performed using articular cartilage explants of pig joints. In acute experimental models of inflammation, 0.5, 1 or 2 mg/kg of seaprose-S was injected intravenously (i.v.) before challenge with inflammatory agents. In adjuvant-induced arthritis, seaprose-S was given as a 2 mg/kg i.v. dose once a day for 4 consecutive days from day 8 after injection of the adjuvant. In cartilage-synovium cocultures, seaprose-S was incubated at a concentration of 0.001 microM and 0.05 microM. Paw volume was measured with a plethysmograph and proteoglycan synthesis was determined in articular cartilage-synovium coculture by incorporation of 35S-sulfate. Seaprose-S inhibited inflammation dose-dependently in carrageenan, concanavalin-A, FeCl2, nystatin-induced paw edema and in carrageenan-induced pleurisy and acetic acid-induced peritonitis. In Freund's adjuvant-induced arthritis, seaprose-S significantly reduced the primary and secondary lesions. In vitro on articular cartilage, seaprose-S increased proteoglycan synthesis in the cartilage alone and reduced the inhibition of proteoglycan synthesis in the cartilage cocultured with minced synovium.
IN THIS ANIMAL STUDY SEAPROSE S WAS SHOWN TO HAVE ANTI-INFLAMMATORY ACTIVITY.
Esch PM, Gerngross H, Fabian A. [Reduction of postoperative swelling. Objective measurement of swelling of the upper ankle joint in treatment with serrapeptase-- a prospective study]. Fortschr Med. 1989 Feb 10;107(4):67-8, 71-2.
[Article in German]
Abstract
Using a quantitative standardized procedure, the swelling of the ankle produced by supination trauma was measured. In the 66 patients with fresh rupture of the lateral ligament treated surgically at our Department between December 1986 and April 1987, a prospective study of the effect of serrapeptase (Aniflazym) SILKWORM! on post-operative swelling and pain was carried out in 3 randomized groups of patients. In the group receiving the test substance, the swelling had decreased by 50% on the third post-operative day, while in the other two control groups (elevation of the leg, bed rest, with and without the application of ice) no reduction in swelling had occurred at that time. The difference is statistically significant (p = 0.013). Decreasing pain correlated for the most part with the reduction in swelling. Thus, the patients receiving the test substance more rapidly became pain-free than did the control groups. On the basis of these results, serrapeptase would appear to be an effective preparation for the post-operative reduction of swelling, in comparison with the classical conservative measures, for example, the application of ice.
SERRAPEPTASE IN ANIFLAYZM IS FROM SILKWORMS, SO THIS STUDY IS NOT APPLICABLE
Selloum L, Bouriche H, Tigrine C, Boudoukha C. Anti-inflammatory effect of rutin on rat paw oedema, and on neutrophils chemotaxis and degranulation. Exp Toxicol Pathol. 2003 Mar;54(4):313-8.
Source
Laboratory of Applied Biochemistry, Department of Biology, Faculty of Sciences, University of Ferhat ABBAS, 19000 Setif, Algeria. laidsel@yahoo.com
Abstract
BACKGROUND:
Rutin, a natural flavone derivative, is known for its pharmacological properties. We have previously reported that this flavonol exerted a potent inhibitory effect on respiratory burst of fMet-Leu-Phe-stimulated neutrophils, as well as on phosphoinositide 3-kinase gamma activity in a cell free system. In the present study, the anti-inflammatory effect of rutin was investigated in vivo and in vitro.
METHODS:
rutin or aspirin (100 mg/kg, body weight) were given orally to rats 1 hour before paw oedema induction, using lambda-carrageenan 1%. The rat paw volume was measured by mean of plethysmometer, initially and during 6 hours. The chemotaxis of neutrophils towards 10(-7) M fMet-Leu-Phe was performed using 48-well chemotaxis chamber. Neutrophils that migrated through 5 microm pore size polycarbonate filter, in presence or in absence of rutin, were counted microscopically. Elastase exocytosis of either phorbol 12-myristate 13-acetate or fMet-Leu-Phe/cytochalasin B-stimulated neutrophils was assessed in absence or in presence of rutin using the synthetic substrate N-Suc-Ala-Ala-Ala-p-nitroanilide. The absorbance of released p-nitroaniline was measured at 405 nm using microplate reader.
RESULTS:
The maximal swelling in placebo group was observed at 5 hours, after lambda-carrageenan injection. Oral administration of rutin reduced rat paw swelling starting 2 hours after lambda-carrageenan injection. Rutin reduced significantly (p < 0.05) and in a dose-dependant manner the polymorphonuclear neutrophils chemotaxis to fMet-Leu-Phe. Furthermore, elastase exocytosis, induced by both stimuli, was partially inhibited by rutin up to 25 microM.
CONCLUSION:
The present study revealed that rutin possesses anti-inflammatory properties.
IN THIS ANIMAL STUDY, ORAL ADMINISTRATION OF RUTIN REDUCED SWELLING WITHIN TWO HOURS.
Guardia T, Rotelli AE, Juarez AO, Pelzer LE. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001 Sep;56(9):683-7.
Source
Departamento de Farmacia, Facultad de Química, Bioquímica y Farmacia, Universidad Nacional de San Luis, Argentina.
Abstract
The anti-inflammatory activities of three flavonoids were investigated in rats using the Mizushima et al. model of acute and chronic inflammation. Intraperitoneal administration of rutin, quercetin (flavonols) and hesperidin (flavanone), given at daily doses equivalent to 80 mg/kg, inhibited both acute and chronic phases of this experimental model of inflammation. Rutin was the most active in the chronic phase.
IN THIS ANIMAL STUDY RUTIN REDUCED INFLAMMATION IN THE ACUTE STAGE, BUT WAS MOST ACTIVE AT THE CHRONIC PHASE OF INFLAMMATION.
Rotelli AE, Guardia T, Juárez AO, de la Rocha NE, Pelzer LE. Comparative study of flavonoids in experimental models of inflammation. Pharmacol Res. 2003 Dec;48(6):601-6.
Source
Cátedra de Farmacología, Facultad de Química, Bioquímica y Farmacia, Universidad Nacional de San Luis, Chacabuco y Pedernera, 5700 San Luis, Argentina. arotelli@unsl.edu.ar
Abstract
The anti-inflammatory activities of flavonols (quercetin, rutin and morin) and flavanones (hesperetin and hesperidin) were investigated in animal models of acute and chronic inflammation. Rutin was only effective in the chronic process, principally in adjuvant arthritis. On neurogenic inflammation induced by xylene, only the flavanones were effective; besides, these compounds were the most effective on subchronic process. The most important compound in reducing paw oedema induced by carrageenan was quercetin.
IN THIS ANIMAL STUDY RUTIN EFFECTIVELY REDUCED INFLAMMATION AT THE CHRONIC PHASE OF INFLAMMATION.
S Sontakke, V Thawani, S Pimpalkhute, P Kabra, S Babhulkar, L Hingorani. Open, randomized, controlled clinical trial of Boswellia serrata extract as compared to valdecoxib in osteoarthritis of knee. Indian Journal of Pharmacology. 2007; 39(1) 27-29
Objective: To compare the efficacy, safety and tolerability of Boswellia serrata extract (BSE) in osteoarthritis (OA) knee with valdecoxib, a selective COX-2 inhibitor. Materials and Methods: In a randomized, prospective, open-label, comparative study the efficacy, safety and tolerability of BSE was compared with valdecoxib in 66 patients of OA of knee for six months. The patients were assessed by WOMAC scale at baseline and thereafter at monthly interval till 1 month after drug discontinuation. Antero-posterior radiographs of affected knee joint were taken at baseline and after 6 months. Results: In BSE group the pain, stiffness, difficulty in performing daily activities showed statistically significant improvement with two months of therapy which even lasted till one month after stopping the intervention. In valdecoxib group the statistically significant improvement in all parameters was reported after one month of therapy but the effect persisted only as long as drug therapy continued. Three patients from BSE group and two from valdecoxib group complained of acidity. One patient from BSE group complained of diarrhea and abdominal cramps. Conclusion: BSE showed a slower onset of action but the effect persisted even after stopping therapy while the action of valdecoxib became evident faster but waned rapidly after stopping the treatment.
SUBJECTS WITH PAINFUL KNEES SHOWED SIGNIFICANT IMPROVEMENT WITH TWO MONTHS OF THERAPY ON BOSWELLIA. DOSE WAS 333MG 3X DAILY. FULL STUDY AVAILABLE HERE: http://www.bioline.org.br/pdf?ph07006
Kimmatkar N, Thawani V, Hingorani L, Khiyani R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee--a randomized double blind placebo controlled trial. Phytomedicine. 2003 Jan;10(1):3-7.
Source
MS Orthopedics, Indira Gandhi Medical College, Nagpur, India.
Abstract
Osteoarthritis is a common, chronic, progressive, skeletal, degenerative disorder, which commonly affects the knee joint. Boswellia serrata tree is commonly found in India. The therapeutic value of its gum (guggulu) has been known. It possesses good anti-inflammatory, anti-arthritic and analgesic activity. A randomized double blind placebo controlled crossover study was conducted to assess the efficacy, safety and tolerability of Boswellia serrata Extract (BSE) in 30 patients of osteoarthritis of knee, 15 each receiving active drug or placebo for eight weeks. After the first intervention, washout was given and then the groups were crossed over to receive the opposite intervention for eight weeks. All patients receiving drug treatment reported decrease in knee pain, increased knee flexion and increased walking distance. The frequency of swelling in the knee joint was decreased. Radiologically there was no change. The observed differences between drug treated and placebo being statistically significant, are clinically relevant. BSE was well tolerated by the subjects except for minor gastrointestinal ADRs. BSE is recommended in the patients of osteoarthritis of the knee with possible therapeutic use in other arthritis.
PATIENTS TREATED WITH BOSWELLIA EXTRACT HAD A DECREASE IN KNEE PAIN AND SWELLING. IT ALSO IMPROVED KNEE FLEXION AND ALLOWED THEM TO INCREASE WALKING DISTANCE. Note: No dosage was given in the abstract however when referenced in another study it was said to be 999 mg. daily. Other study available here
Sengupta K, Alluri KV, Satish AR, Mishra S, Golakoti T, Sarma KV, Dey D, Raychaudhuri SP. A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin for treatment of osteoarthritis of the knee. Arthritis Res Ther. 2008;10(4):R85
Source
Cellular and Molecular Biology Division, Laila Impex R&D Center, Jawahar Autonagar, Vijayawada, India.
Abstract
INTRODUCTION:
5-Loxin is a novel Boswellia serrata extract enriched with 30% 3-O-acetyl-11-keto-beta-boswellic acid (AKBA), which exhibits potential anti-inflammatory properties by inhibiting the 5-lipoxygenase enzyme. A 90-day, double-blind, randomized, placebo-controlled study was conducted to evaluate the efficacy and safety of 5-Loxin in the treatment of osteoarthritis (OA) of the knee.
METHODS:
Seventy-five OA patients were included in the study. The patients received either 100 mg (n = 25) or 250 mg (n = 25) of 5-Loxin daily or a placebo (n = 25) for 90 days. Each patient was evaluated for pain and physical functions by using the standard tools (visual analog scale, Lequesne's Functional Index, and Western Ontario and McMaster Universities Osteoarthritis Index) at the baseline (day 0), and at days 7, 30, 60 and 90. Additionally, the cartilage degrading enzyme matrix metalloproteinase-3 was also evaluated in synovial fluid from OA patients. Measurement of a battery of biochemical parameters in serum and haematological parameters, and urine analysis were performed to evaluate the safety of 5-Loxin in OA patients.
RESULTS:
Seventy patients completed the study. At the end of the study, both doses of 5-Loxin conferred clinically and statistically significant improvements in pain scores and physical function scores in OA patients. Interestingly, significant improvements in pain score and functional ability were recorded in the treatment group supplemented with 250 mg 5-Loxin as early as 7 days after the start of treatment. Corroborating the improvements in pain scores in treatment groups, we also noted significant reduction in synovial fluid matrix metalloproteinase-3. In comparison with placebo, the safety parameters were almost unchanged in the treatment groups.
CONCLUSION:
5-Loxin reduces pain and improves physical functioning significantly in OA patients; and it is safe for human consumption. 5-Loxin may exert its beneficial effects by controlling inflammatory responses through reducing proinflammatory modulators, and it may improve joint health by reducing the enzymatic degradation of cartilage in OA patients.
100-250 MG OF 5-LOXIN, A BOSWELLIA EXTRACT, REDUCED INFLAMMATORY RESPONSE AND CARTILAGE DEGRADATION WHILE IMPROVING PAIN AND PHYSICAL FUNCTION SCORES. USE OF THIS STUDY WOULD DEPEND UPON THE TYPE OF EXTRACT YOU ARE USING, AND MAY NOT APPLY.
A. Sharma, S. Bhatia, M. D. Kharya, V. Gajbhiye, N Ganesh, A. G. Namdeo, K. R. Mahadik. Anti-inflammatory and analgesic activity of different fractions of Boswellia serrata. Int Jnl of Phytomedicine. 2010 V 2 No 1
Abstract
The study was designed to investigate the anti-inflammatory and analgesic effect of different fractions of Boswellia serrata. The effect of different fractions of Boswellia serrata were studied using carrageenan induced paw edema, acetic acid induced writhing response, formalin induced pain, hot plate and tail flick method for studying anti-inflammatory and analgesic activity, respectively. The different fractions of B. serrata, essential oil (10 ml/kg), gum (100 mg/kg, resin (100 mg/kg) oleo-resin (100 mg/kg) and oleo-gum-resin (100 mg/kg) significantly reduces carrageenan induced inflammation in rats and shows analgesic activity, as determined by acetic acid induced writhing response, formalin induced pain, hot plate and tail flick method. The different fractions of B. serrata showed prompt anti-inflammatory and analgesic activity due to the inhibition of 5-lipoxygenase enzyme.
IN THIS ANIMAL STUDY BOSWELLIA REDUCED INFLAMMATION IN RATS.
Kuptniratsaikul V, Thanakhumtorn S, Chinswangwatanakul P, Wattanamongkonsil L, Thamlikitkul V. Efficacy and safety of Curcuma domestica extracts in patients with knee osteoarthritis. J Altern Complement Med. 2009 Aug;15(8):891-7.
Source
Department of Rehabilitation Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand. sivth@mahidol.ac.th
Abstract
OBJECTIVE:
The objective of this study was to determine the efficacy and safety of Curcuma domestica extracts in pain reduction and functional improvement in patients with knee osteoarthritis.
STUDY DESIGN AND SETTING:
The design and setting were a randomized controlled study at a university hospital in Bangkok, Thailand.
METHODS:
One-hundred and seven (107) patients with primary knee osteoarthritis (OA) with pain score of > or =5 were randomized to receive ibuprofen 800 mg per day or C. domestica extracts 2 g per day for 6 weeks. The main outcomes were improvement in pain on level walking, pain on stairs, and functions of knee assessed by time spent during 100-m walk and going up and down a flight of stairs. The adverse events were also recorded.
RESULTS:
Fifty-two (52) and 55 patients were randomized to C. domestica extracts and ibuprofen groups, respectively. Baseline characteristics of the patients in both groups were not different. The mean scores of the aforementioned outcomes at weeks 0, 2, 4, and 6 were significantly improved when compared with the baseline values in both groups. There was no difference in those parameters between the patients receiving ibuprofen and C. domestica extracts, except pain on stairs (p = 0.016). No significant difference of adverse events between both groups was found (33.3% versus 44.2%, p = 0.36 in C. domestica extracts and ibuprofen groups, respectively).
CONCLUSIONS:
C. domestica extracts seem to be similarly efficacious and safe as ibuprofen for the treatment of knee OA.
CANNOT USE/DOSAGE
Olas B, Wachowicz B, Nowak P, Stochmal A, Oleszek W, Glowacki R, Bald E. Comparative studies of the antioxidant effects of a naturally occurring resveratrol analogue -- trans-3,3',5,5'-tetrahydroxy-4'-methoxystilbene and resveratrol -- against oxidation and nitration of biomolecules in blood platelets. Cell Biol Toxicol. 2008 Aug;24(4):331-40. Source
Department of General Biochemistry, Institute of Biochemistry, University of Lodz, Lodz, Poland. olasb@biol.uni.lodz.pl
Abstract
The action of two phenolic compounds isolated from the bark of Yucca schidigera: trans-3,3',5,5'-tetrahydroxy-4'-methoxystilbene and its analogue -- resveratrol (trans-3,4',5-trihydroxystilbene, present also in grapes and wine) on oxidative/nitrative stress induced by peroxynitrite (ONOO(-), which is strong physiological oxidant and inflammatory mediator) in human blood platelets was compared. The trans-3,3',5,5'-tetrahydroxy-4'-methoxystilbene, like resveratrol, significantly inhibited protein carbonylation and nitration (measured by enzyme-linked immunosorbent assay method) in the blood platelets treated with peroxynitrite (0.1 mM) and markedly reduced an oxidation of thiol groups of proteins (estimated with 5,5'-dithio-bis(2-nitro-benzoic acid)] or glutathione (measured by high performance liquid chromatography method) in these cells. The trans-3,3',5,5'-tetrahydroxy-4'-methoxystilbene, like resveratrol, also caused a distinct reduction of platelet lipid peroxidation induced by peroxynitrite. The obtained results indicate that in vitro trans-3,3',5,5'-tetrahydroxy-4'-methoxystilbene and resveratrol have very similar protective effects against peroxynitrite-induced oxidative/nitrative damage to the human platelet proteins and lipids. Moreover, trans-3,3',5,5'-tetrahydroxy-4'-methoxystilbene proved to be even more potent than resveratrol in antioxidative tests. We conclude that the novel tested phenolic compound -- trans-3,3',5,5'-tetrahydroxy-4'-methoxystilbene isolated from Y. schidigera bark possessing Generally Recognized As Safe label given by the Food and Drug Administration and allows their human dietary use -- seems to be a promising candidate for future evaluations of its antioxidative activity and may be a good candidate for scavenging peroxynitrite.
YUCCA SCHIDIGERA BARK MAY REDUCE INFLAMMATORY OXIDATIVE STRESS INDUCED BY PEROXYNITRITE
Piacente S, Montoro P, Oleszek W, Pizza C. Yucca schidigera bark: phenolic constituents and antioxidant activity. J Nat Prod. 2004 May;67(5):882-5.
Source
Dipartimento di Scienze Farmaceutiche, Università degli Studi di Salerno, Via Ponte Don Melillo, 84084 Fisciano, Salerno, Italy.
Abstract
Two new phenolic constituents with unusual spirostructures, named yuccaols D (1) and E (2), were isolated from the MeOH extract of Yucca schidigera bark. Their structures were established by spectroscopic (ESIMS and NMR) analysis. The new yuccaols D and E, along with resveratrol (3), trans-3,3',5,5'-tetrahydroxy-4'-methoxystilbene (4), yuccaols A-C (5-7), yuccaone A (8), larixinol (9), the MeOH extract of Yucca schidigera bark, and the phenolic portion of this extract, were assayed for antioxidant activity by measuring the free radical scavenging effects using two different assays, namely, the Trolox Equivalent Antioxidant Capacity (TEAC) assay and the coupled oxidation of beta-carotene and linoleic acid (autoxidation assay). The significant activities exhibited by the phenolic fraction and its constituents in both tests show the potential use of Y. schidigera as a source of antioxidant principles.
YUCCA SCHIDIGERA BARK DEMONSTRATES ANTIOXIDANT ACTIVITY.
Chantre P, Cappelaere A, Leblan D, Guedon D, Vandermander J, Fournie B. Efficacy and tolerance of Harpagophytum procumbens versus diacerhein in treatment of osteoarthritis. Phytomedicine. 2000 Jun;7(3):177-83.
Source
Laboratoires Arkopharma, Carros, France.
Abstract
In a double-blind, randomized, multicentre clinical study, the efficacy and tolerance of a herbal medicine product, Harpadol (6 capsules/day, each containing 435 mg of powdered cryoground powder Harpagophytum procumbens), was compared with diacerhein 100 mg/day in the treatment, for 4 months, of 122 patients suffering from osteoarthritis of the knee and hip. Assessments of pain and functional disability were made on a 10 cm horizontal visual analogue scale; severity of osteoarthritis was evaluated by Lequesne's index. Spontaneous pain showed a significant improvement during the course of the study and there was no difference in the efficacy of the two treatments. Similarly, there was a progressive and significant reduction in the Lequesne functional index and no statistical difference was found between Harpadol and diacerhein. At completion of the study, patients taking Harpadol were using significantly less NSAIDs and antalgic drugs. The frequency of adverse events was significantly lower in the Harpadol group. The most frequent event reported was diarrhea, occurring in 8.1% and 26.7% of Harpadol and diacerhein patients respectively. The global tolerance assessment by patients at the end of treatment favoured Harpadol. The results of this study demonstrate that Harpadol is comparable in efficacy and superior in safety to diacerhein.
CANNOT USE/DOSAGE
Chrubasik S, Junck H, Breitschwerdt H, Conradt C, Zappe H. Effectiveness of Harpagophytum extract WS 1531 in the treatment of exacerbation of low back pain: a randomized, placebo-controlled, double-blind study. Eur J Anaesthesiol. 1999 Feb;16(2):118-29.
Source
Department of Medical Biometry, University of Heidelberg, Germany.
Abstract
Two daily doses of oral Harpagophytum extract WS 1531 (600 and 1200, respectively, containing 50 and 100 mg of the marker harpagoside) were compared with placebo over 4 weeks in a randomized, double-blind study in 197 patients with chronic susceptibility to back pain and current exacerbations that were producing pain worse than 5 on a 0-10 visual analogue scale. The principal outcome measure, based on pilot studies, was the number of patients who were pain free without the permitted rescue medication (tramadol) for 5 days out of the last week. The treatment and placebo groups were well matched in physical characteristics, in the severity of pain, duration, nature and accompaniments of their pain, the Arhus low back pain index and in laboratory indices of organ system function. A total of 183 patients completed the study. The numbers of pain-free patients were three, six and 10 in the placebo group (P), the Harpagophytum 600 group (H600) and the Harpagophytum 1200 group (H1200) respectively (P = 0.027, one-tailed Cochrane-Armitage test). The majority of responders' were patients who had suffered less than 42 days of pain, and subgroup analyses suggested that the effect was confined to patients with more severe and radiating pain accompanied by neurological deficit. However, subsidiary analyses, concentrating on the current pain component of the Arhus index, painted a slightly different picture, with the benefits seeming, if anything, to be greatest in the H600 group and in patients without more severe pain, radiation or neurological deficit. Patients with more pain tended to use more tramadol, but even severe and unbearable pain would not guarantee that tramadol would be used at all, and certainly not to the maximum permitted dose. There was no evidence for Harpagophytum-related side-effects, except possibly for mild and infrequent gastrointestinal symptoms.
Heal-n-Soothe® CONTAINS DEVILS CLAW, BUT IT IS UNCLEAR WHAT THE LEVEL OF HARPAGOSIDES ARE. 50-100 MG HARAGOSIDES WERE USED IN THIS STUDY AND WORKED BETTER THAN PLACEBO.
Gagnier JJ, van Tulder M, Berman B, Bombardier C. Herbal medicine for low back pain. Cochrane Database Syst Rev. 2006 Apr 19;(2):CD004504.
Source
Provincal Medical Centre, 5955 Ontario St., Unit 307, Windsor, Ontario, Canada, N8S1W6. j.gagnier@utoronto.ca
Abstract
BACKGROUND:
Low-back pain is a common condition and a substantial economic burden in industrialized societies. A large proportion of patients with chronic low-back pain use complementary and alternative medicine (CAM), visit CAM practitioners, or both. Several herbal medicines have been purported for use in low-back pain.
OBJECTIVES:
To determine the effectiveness of herbal medicine for non-specific low-back pain.
SEARCH STRATEGY:
We searched the following electronic databases: Cochrane Complementary Medicine Field Trials Register (Issue 3, 2005), MEDLINE (1966 to July 2005), EMBASE (1980 to July 2005); checked reference lists in review articles, guidelines and retrieved trials; and personally contacted individuals with expertise in this very specialized area.
SELECTION CRITERIA:
We included randomized controlled trials, examining adults (over 18 years of age) suffering from acute, sub-acute or chronic non-specific low-back pain. The interventions were herbal medicines, defined as plants that are used for medicinal purposes in any form. Primary outcome measures were pain and function.
DATA COLLECTION AND ANALYSIS:
Two authors (JJG & MVT) conducted the database searches. One author contacted content experts and acquired relevant citations. Full references and abstracts of the identified studies were downloaded. A hard copy was retrieved for final inclusion decisions. Methodological quality and clinical relevance were assessed separately by two individuals. Disagreements were resolved by consensus.
MAIN RESULTS:
Ten trials were included in this review. Two high quality trials examining the effects of Harpagophytum Procumbens (Devil's Claw) found strong evidence that daily doses standardized to 50 mg or 100 mg harpagoside were better than placebo for short-term improvements in pain and rescue medication. Another high quality trial demonstrated relative equivalence to 12.5 mg per day of rofecoxib (Vioxx). Two trials examining the effects of Salix Alba (White Willow Bark) found moderate evidence that daily doses standardized to 120 mg or 240 mg salicin were better than placebo for short-term improvements in pain and rescue medication. An additional trial demonstrated relative equivalence to 12.5 mg per day of rofecoxib. Three low quality trials on Capsicum Frutescens (Cayenne), examining various topical preparations, found moderate evidence that Capsicum Frutescens produced more favourable results than placebo and one trial found equivalence to a homeopathic ointment.
AUTHORS' CONCLUSIONS:
Harpagophytum Procumbens, Salix Alba and Capsicum Frutescens seem to reduce pain more than placebo. Additional trials testing these herbal medicines against standard treatments are needed. The quality of reporting in these trials was generally poor. Trialists should refer to the CONSORT statement extension for reporting trials of herbal medicine interventions.
Heal-n-Soothe® CONTAINS DEVILS CLAW, BUT IT IS UNCLEAR WHAT THE LEVEL OF HARPAGOSIDES ARE. 50-100 MG HARAGOSIDES WERE USED IN THESE STUDIES AND WORKED BETTER THAN PLACEBO.
REFERENCE NOTE ABOUT DEVIL'S CLAW: Devil's claw has been studied for low back pain, muscle pain, and osteoarthritis using daily doses of crude tuber up to 9 g daily, 1 to 3 g of extract, and 50 to 100 mg of harpagoside. Standardized preparations include LI 174 ( Rivoltan ), Doloteffin (more than 50 mg harpagoside), and WS 1531. A level of more than 1% harpagoside in root is considered acceptable. Commercial sources of devil's claw extract contain 1.4% to 2% harpagoside.
Göbel H, Heinze A, Ingwersen M, Niederberger U, Gerber D. [Effects of Harpagophytum procumbens LI 174 (devil's claw) on sensory, motor und vascular muscle reagibility in the treatment of unspecific back pain]. Schmerz. 2001 Feb;15(1):10-8.
[Article in German]
Source
Neurologisch-verhaltensmedizinische, Schmerzklinik Kiel in Kooperation mit der Universität Kiel. h.gobel@neurologie.uni-kiel.de
Abstract
PROBLEM:
This randomised, double-blind, placebo controlled study was intended to investigate the effects of Harpagophytum procumbens (Devil's Claw) on sensory, motor and vascular mechanisms of muscle pain. In addition to clinical efficacy and tolerability, possible action mechanisms were analysed by means of experimental algesimetric methods.
METHODOLOGY:
The study was performed on patients with slight to moderate muscular tension or slight muscular pain of the back, shoulder and neck. On a double-blind randomised basis the verum group received 2x1 film tablets per day, i. e. 2x480 mg/day, of Harpagophytum extract LI 174 (Rivoltan(R)) at 8.00 a.m. and 8.00 p.m. over a certain period. The duration of the therapy was 4 weeks. Data recording at 14-day intervals was made using a visual analogue scale, pressure algometer test, recording of antinociceptive muscular reflexes, muscle stiffness test, EMG surface activity, muscular ischaemia test, clinical global score and subjective patient and physician ratings.
RESULTS:
A total of 31 patients in the verum group and 32 in the placebo group were treated. After four weeks of treatment there was found to be a clear clinical efficacy of the verum on the clinical global score and in the patient and physician ratings. Highly significant effects were found in the visual analogue scale, the pressure algometer test, the muscle stiffness test and the muscular ischaemia test. No difference from placebo was found in the recording of antinociceptive muscular reflexes or in the EMG surface activity. Tolerability was good; no serious adverse effects occurred.
CONCLUSIONS:
A highly significant clinical efficacy was achieved with a monotherapy of Harpagophytum dry extract LI 174 after four weeks' treatment at a dosage of 2x480 mg/day in cases of slight to moderate muscular pain. With regard to the action mechanisms investigated, it may be concluded that treatment with Harpagophytum extract LI 174 may be expected to have a significant influence on sensory and vascular muscular response and bring about a reduction in muscle stiffness. No central nervous effects were discovered.
CANNOT USE/DOSAGE
Leblan D, Chantre P, Fournié B. Harpagophytum procumbens in the treatment of knee and hip osteoarthritis. Four-month results of a prospective, multicenter, double-blind trial versus diacerhein. Joint Bone Spine. 2000;67(5):462-7.
Source
Laboratoires Arkopharma, Carros, France.
Abstract
OBJECTIVE:
To evaluate the efficacy and safety of Harpagophytum in the treatment of hip and knee osteoarthritis comparatively with the slow-acting drug for osteoarthritis, diacerhein.
PATIENTS AND METHODS:
A multicenter, randomized, double-blind, parallel-group study was conducted in 122 patients with hip and/or knee osteoarthritis. Treatment duration was four months and the primary evaluation criterion was the pain score on a visual analog scale. Harpagophytum 2,610 mg per day was compared with diacerhein 100 mg per day.
RESULTS:
After four months, considerable improvements in osteoarthritis symptoms were seen in both groups, with no significant differences for pain, functional disability, or the Lequesne score. However, use of analgesic (acetaminophen-caffeine) and nonsteroidal anti-inflammatory (diclofenac) medications was significantly reduced in the Harpagophytum group, which also had a significantly lower rate of adverse events.
CONCLUSION:
In this study, Harpagophytum was at least as effective as a reference drug (diacerhein) in the treatment of knee or hip osteoarthritis and reduced the need for analgesic and nonsteroidal anti-inflammatory therapy.
CANNOT USE/DOSAGE
Sasaki T, Kojima S. Ga uptake and heparan sulfate content of Ehrlich solid tumor in mice. Eur J Nucl Med. 1986;12(4):182-6.
Abstract
The relationship between 67Ga uptake and heparan sulfate (HS) content in Ehrlich solid tumor (EST) of mice was investigated, and the effect of cyanomethylamine, papain, streptozotocin, or bleomycin pretreatment on 67Ga uptake in EST was studied. 67Ga uptakes in EST and kidney were much higher than other tissues, and these tissues also contained large amounts of HS. 67Ga uptakes and HS synthesis in the EST were inhibited by pretreatment with cyanomethylamine or papain (inhibitors of fibrosis). Parallel reductions of 67Ga uptake and HS synthesis in EST were observed in EST transplanted into streptozotocin-induced diabetic mice. The weight of EST in the bleomycin-injected group was decreased to less than half of the control, but no effect was observed on 67Ga uptake per gram of EST. These results suggest that 67Ga uptake in the tumor and inflammatory lesions are related to the quantity of HS in these tissues, and the correlation between the uptake of 67Ga and the rate of cellular proliferation is secondary.
PAPAIN IS AN INHIBITOR OF FIBROSIS BUT COULD NOT FIND HUMAN STUDIES TO SUPPORT SAME.
Skyrme-Jones RA, O'Brien RC, Berry KL, Meredith IT. Vitamin E supplementation improves endothelial function in type I diabetes mellitus: a randomized, placebo-controlled study. J Am Coll Cardiol. 2000 Jul;36(1):94-102.
Source
Centre for Heart and Chest Research, Monash Medical Centre and Monash University, Melbourne, Australia.
Abstract
OBJECTIVES:
We sought to determine, in a double-blind, placebo-controlled, randomized study, whether vitamin E supplementation (1,000 IU for three months) would improve impaired conduit and resistance vessel endothelial vasodilator function (EVF) and systemic arterial compliance (SAC) in type I diabetes mellitus (DM).
BACKGROUND:
Oxidative stress is thought to be important in the pathogenesis of impaired EVF. Consistent with this hypothesis, we have recently shown that impaired EVF is related to low density lipoprotein (LDL) vitamin E content (VEC) in young subjects with type 1 DM.
METHODS:
We assessed EVF in the brachial artery (using noninvasive ultrasound, flow-mediated vasodilation [FMD]; n = 41) and in the forearm resistance vessels (by flow responses to intrabrachial acetylcholine [ACh]; n = 21) and measured SAC (simultaneous aortic blood flow and carotid pressure measurements; n = 41) before and after active or placebo therapy.
RESULTS:
The LDL VEC was increased by 127% after supplementation, resulting in a significant reduction in the oxidative susceptibility of LDL. There was no time-dependent change in FMD or in the response to ACh or SAC in the placebo group. A significant improvement in FMD (2.6 +/- 0.6% to 7.0 +/- 0.7%, p < 0.005) and the dose response to ACh (p < 0.05) were observed in those randomized to vitamin E therapy. Systemic arterial compliance was not affected by vitamin E (0.41 +/- 0.03 vs. 0.49 +/- 0.06 arbitrary compliance units, p = NS). The change in FMD was related to the change in LDL VEC (r = 0.42, p < 0.05) and the change in the oxidative susceptibility of LDL (r = 0.64, p < 0.0001).
CONCLUSIONS:
Short-term daily oral supplementation with vitamin E improves EVF in both the conduit and resistance vessels of young subjects with type I DM.
CANNOT USE/DOSAGE
Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr. 2007 Oct;137(10):2171-84.
Source
Department of Biology, McMaster University, Hamilton, Ontario, Canada L8S 4K1.
Abstract
Vitamin C is required for collagen synthesis and biosynthesis of certain hormones and recommended dietary intake levels are largely based these requirements. However, to function effectively as an antioxidant (or a pro-oxidant), relatively high levels of this vitamin must be maintained in the body. The instability of vitamin C combined with its relatively poor intestinal absorption and ready excretion from the body reduce physiological availability of this vitamin. This inability to maintain high serum levels of vitamin C may have serious health implications and is particularly relevant in the onset and progression of degenerative disease, such as cancer and cardiovascular disease (CVD), which have a strong contributing oxidative damage factor. In this review, we examine recent studies on the regulation of transport mechanisms for vitamin C, related clinical ramifications, and potential implications in high-dose vitamin C therapy. We also evaluate recent clinical and scientific evidence on the effects of this vitamin on cancer and CVD, with focus on the key mechanisms of action that may contribute to the therapeutic potential of this vitamin in these diseases. Several animal models that could be utilized to address unresolved questions regarding the feasibility of vitamin C therapy are also discussed.
Singh RK, Rai D, Yadav D, Bhargava A, Balzarini J, De Clercq E. Synthesis, antibacterial and antiviral properties of curcumin bioconjugates bearing dipeptide, fatty acids and folic acid. Eur J Med Chem. 2010 Mar;45(3):1078-86. Epub 2010 Jan 19.
Source
Nucleic Acids Research Laboratory, Department of Chemistry, University of Allahabad, Allahabad 211002, India.
Abstract
Curcumin bioconjugates, viz. di-O-tryptophanylphenylalanine curcumin (2), di-O-decanoyl curcumin (3), di-O-pamitoyl curcumin (4), di-O-bis-(gamma,gamma)folyl curcumin (6), C(4)-ethyl-O-gamma-folyl curcumin (8) and 4-O-ethyl-O-gamma-folyl curcumin (10) have been synthesized and tested for their antibacterial and antiviral activities. The conjugates 2, 3, 4, 6 and 8 have shown very promising antibacterial activity with MIC ranging between 0.09 and 0.67 microM against Gram-positive cocci and Gram-negative bacilli. Further, the conjugates 2, 3, 6, 8 and 10 have been screened for their antiviral activities against HSV, VSV, FIPV, PIV-3, RSV and FHV and the molecules 2 and 3 have shown good results with EC(50) 0.011 microM and 0.029 microM against VSV and FIPV/FHV, respectively. However, the molecules did not show expected results against HIV-1 III(B) and ROD strains in MTT assay.
Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006 Mar 27;78(18):2081-7. Epub 2006 Jan 18.
Source
Department of Pathology, Uniformed Services University of the Life Sciences, Center for Combat Casualty and Life Sustainment Research, Bethesda, Maryland 20814, USA. maheshwari@usuhs.mil
Abstract
Turmeric (Curcuma longa rhizomes), commonly used as a spice is well documented for its medicinal properties in Indian and Chinese systems of medicine. It has been widely used for the treatment of several diseases. Epidemiological observations, though inconclusive, are suggestive that turmeric consumption may reduce the risk of some form of cancers and render other protective biological effects in humans. These biological effects of turmeric have been attributed to its constituent curcumin that has been widely studied for its anti-inflammatory, anti-angiogenic, anti-oxidant, wound healing and anti-cancer effects. As a result of extensive epidemiological, clinical, and animal studies several molecular mechanisms are emerging that elucidate multiple biological effects of curcumin. This review summarizes the most interesting in vitro and in vivo studies on the biological effects of curcumin.
WARNING: If you are pregnant, lactating, a hemophiliac, have an ulcer, are taking blood thinners or anti-coagulants (such as Coumadin, Heparin and Plavix), do not take Heal-n-Soothe without the supervision of your doctor or healthcare provider. Discontinue use 2 weeks prior to surgery.
Please Note: Persons who suffer from medical conditions or who are taking medications should consult their physician prior to taking this product. This product may thin the blood and may not be appropriate for all persons. Do not take this product if you know or suspect that you are allergic to pineapple, papaya, or any ingredients in this product. As with all dietary supplements, those who are pregnant or nursing should consult their physician prior to taking this product.
*These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease. If you are pregnant, nursing, taking medication, or have a medical condition, consult your physician before using this product.